Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation
Abstract
:1. Introduction
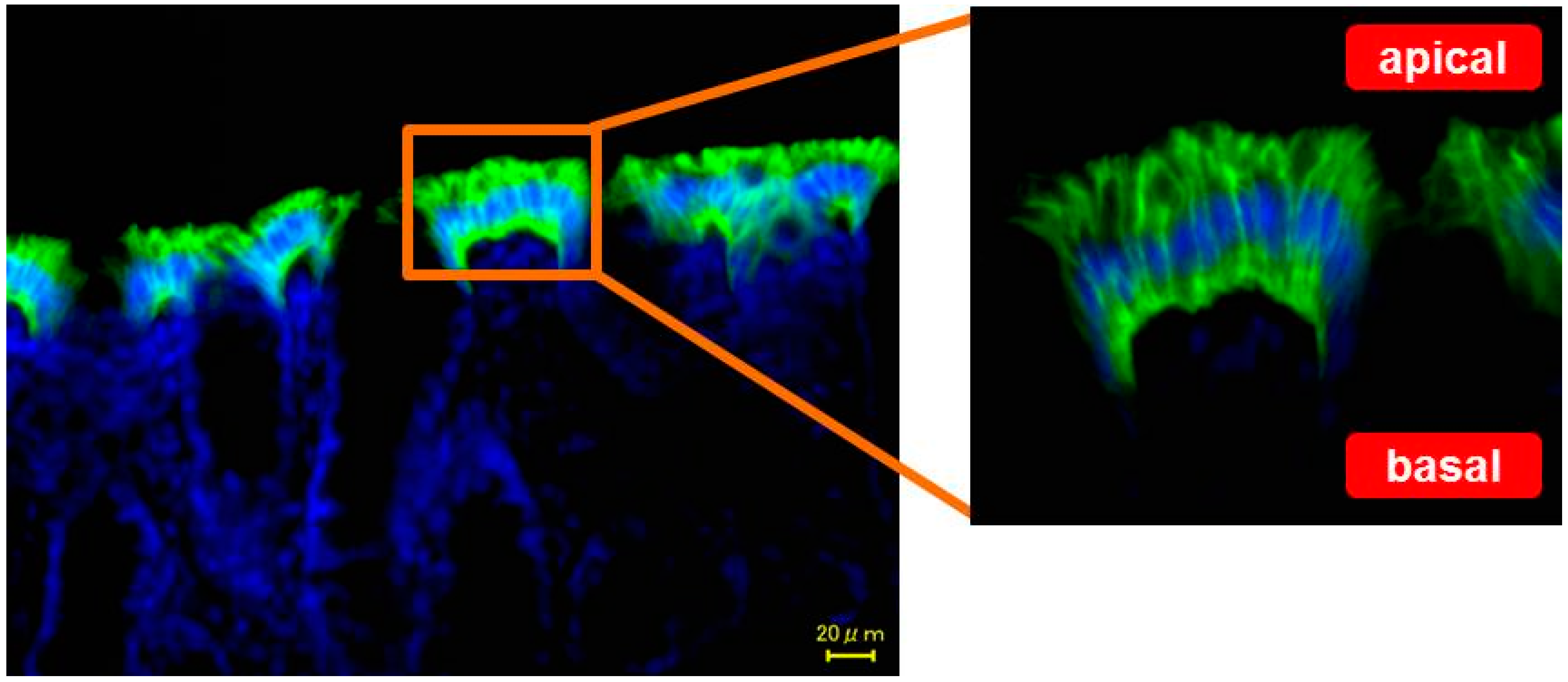
2. Localization of AQP3 in the Colon
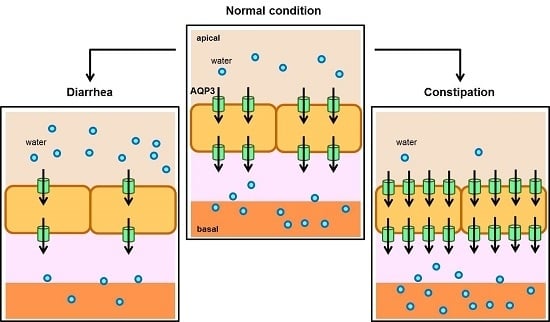
3. Relation between AQP3 Expression and Diarrhea
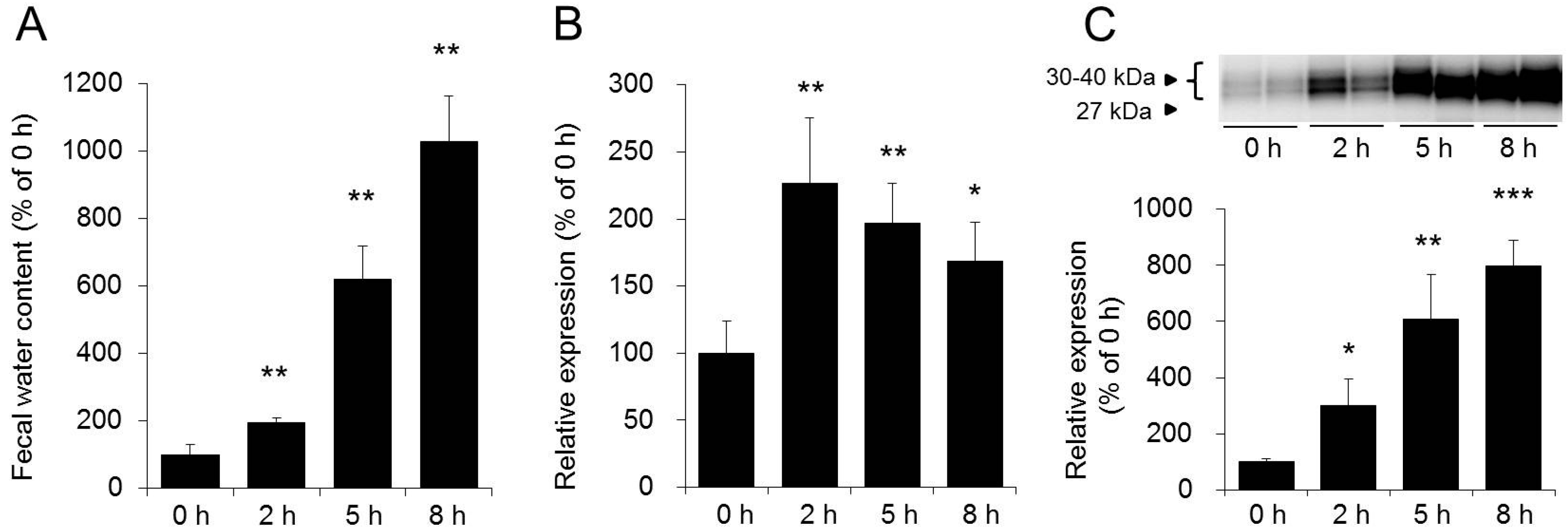
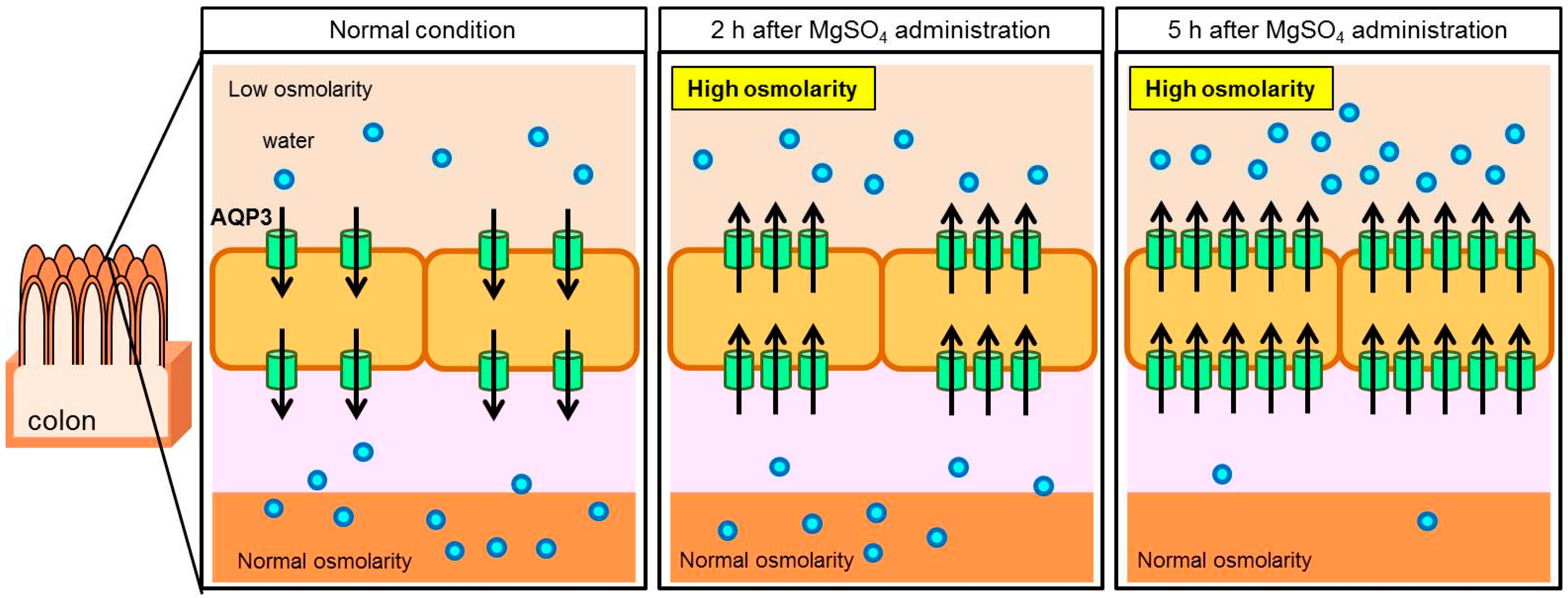
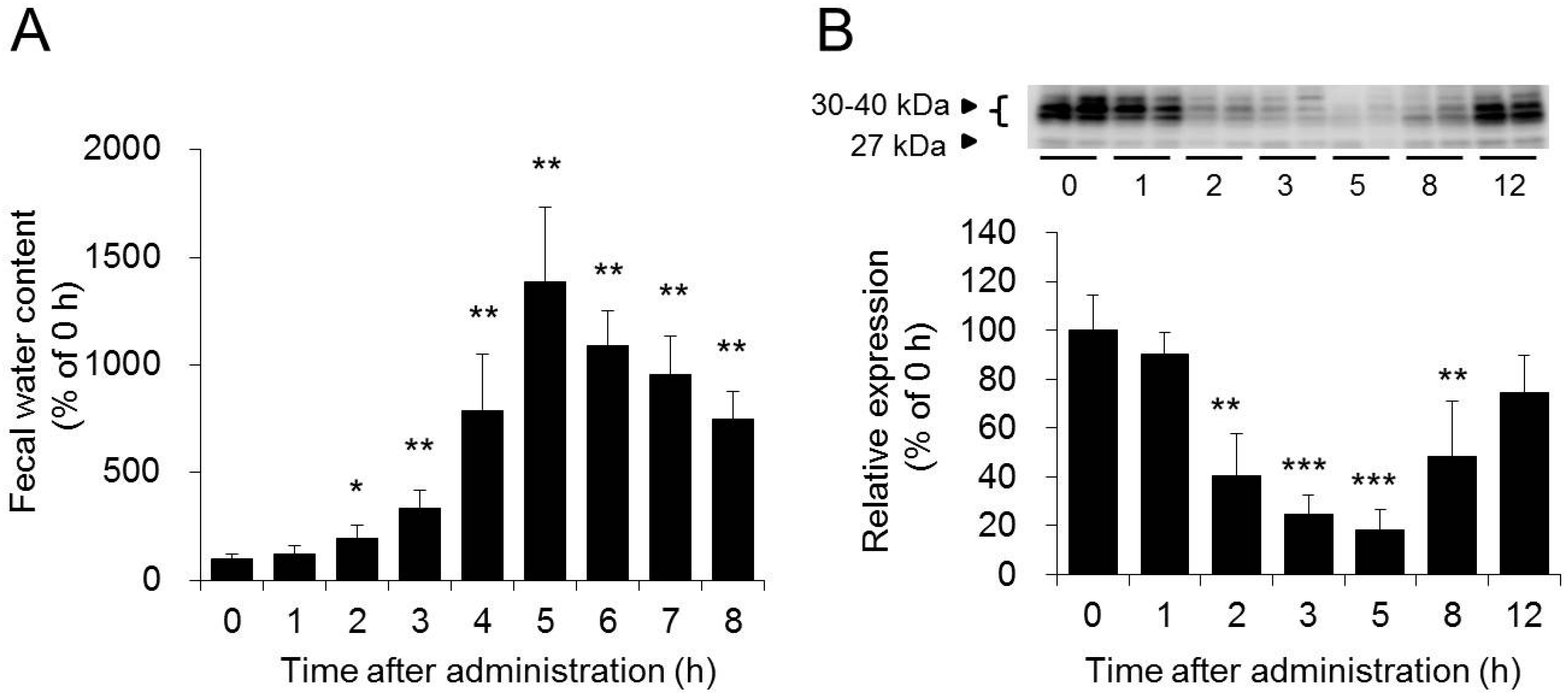
3.1. Role of AQP3 in the Colon in the Laxative Effect of Magnesium Sulfate
3.2. Role of AQP3 in the Colon in the Laxative Effects of Bisacodyl and Sennoside A
4. Relation between AQP3 Expression and Constipation
5. AQP and Clinical Application
5.1. Role of AQP3 in the Colon in the Concomitant Use of Laxatives
5.2. Efficacy of Laxatives on Morphine-Induced Constipation and AQP3 in the Colon
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP | aquaporin |
| CREB | cAMP response element binding protein |
| PGE2 | prostaglandin E2 |
| COX-2 | cyclooxygenase-2 |
| TNF-α | tumor necrosis factor-α |
| SERT | serotonin reuptake transporter |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| NSAIDs | non-steroidal anti-inflammatory drugs |
References
- Hall, K.E.; Proctor, D.D.; Fisher, L.; Rose, S. American gastroenterological association future trends committee report: Effects of aging of the population on gastroenterology practice, education, and research. Gastroenterology 2005, 129, 1305–1338. [Google Scholar] [CrossRef] [PubMed]
- Quigley, C. The role of opioids in cancer pain. BMJ 2005, 331, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, A.; McMillan, S.C.; Zenerino, F.; Rattone, V.; Grubich, S.; Guazzini, A.; Rasero, L. Validity and reliability of the italian constipation assessment scale. Int. J. Palliat. Nurs. 2012, 18, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Pitchumoni, C.S. Gastrointestinal side effects of prescription medications in the older adult. J. Clin. Gastroenterol. 2009, 43, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.D.; Wright, E.M.; Zeuthen, T. Water pumps. J. Physiol. 2002, 542, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Physiological roles of glycerol-transporting aquaporins: The aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Lian, S.C.; Finkbeiner, W.E.; Verkman, A.S. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am. J. Physiol. 1994, 266, C893–C903. [Google Scholar] [PubMed]
- Ishibashi, K.; Sasaki, S.; Saito, F.; Ikeuchi, T.; Marumo, F. Structure and chromosomal localization of a human water channel (AQP3) gene. Genomics 1995, 27, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Ishibashi, K.; Kuwahara, M.; Inase, N.; Ichioka, M.; Sasaki, S.; Marumo, F. Cloning and functional expression of human aquaporin8 cDNA and analysis of its gene. Genomics 1998, 54, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Yamamoto, T.; Tani, T.; Nihei, K.; Kondo, D.; Funaki, H.; Yaoita, E.; Kawasaki, K.; Sato, N.; Hatakeyama, K.; et al. Expression and localization of aquaporins in rat gastrointestinal tract. Am. J. Physiol. 1999, 276, C621–C627. [Google Scholar] [PubMed]
- Silberstein, C.; Kierbel, A.; Amodeo, G.; Zotta, E.; Bigi, F.; Berkowski, D.; Ibarra, C. Functional characterization and localization of AQP3 in the human colon. Braz. J. Med. Biol. Res. 1999, 32, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, P.; Cid, L.P.; Vio, C.P.; Sepulveda, F.V. Aquaporin-2, a regulated water channel, is expressed in apical membranes of rat distal colon epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G856–G863. [Google Scholar] [PubMed]
- Hardin, J.A.; Wallace, L.E.; Wong, J.F.; O’Loughlin, E.V.; Urbanski, S.J.; Gall, D.G.; MacNaughton, W.K.; Beck, P.L. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res. 2004, 318, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Tajika, Y.; Ablimit, A.; Aoki, T.; Hagiwara, H.; Takata, K. Aquaporins in the digestive system. Med. Electron. Microsc. 2004, 37, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Hou, X.H. Expression of aquaporin 8 in colonic epithelium with diarrhoea-predominant irritable bowel syndrome. Chin. Med. J. 2007, 120, 313–316. [Google Scholar] [PubMed]
- Zahn, A.; Moehle, C.; Langmann, T.; Ehehalt, R.; Autschbach, F.; Stremmel, W.; Schmitz, G. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J. Gastroenterol. 2007, 13, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Ma, T.; Filiz, F.; Verkman, A.S.; Bastidas, J.A. Colon water transport in transgenic mice lacking aquaporin-4 water channels. Am. J. Physiol. Gastrointest Liver Physiol. 2000, 279, G463–G470. [Google Scholar] [PubMed]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Asp. Med. 2012, 33, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J. Gastroenterol. Hepatol. 2003, 18, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rai, T.; Sasaki, S.; Uchida, S. Polarized trafficking of the aquaporin-3 water channel is mediated by an NH2-terminal sorting signal. Am. J. Physiol. Cell Physiol. 2006, 290, C298–C304. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Mazzone, A.; Bizzoca, A.; Cavalier, A.; Cassano, G.; Thomas, D.; Svelto, M. Expression and immunolocalization of the aquaporin-8 water channel in rat gastrointestinal tract. Eur. J. Cell Biol. 2001, 80, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Cova, E.; Gastaldi, G.; Tritto, S.; Grazioli, M.; LaRusso, N.F.; Splinter, P.L.; D’Adamo, P.; Tosco, M.; Ventura, U. Aquaporin-8 is involved in water transport in isolated superficial colonocytes from rat proximal colon. J. Nutr. 2005, 135, 2329–2336. [Google Scholar] [PubMed]
- Laforenza, U.; Gastaldi, G.; Grazioli, M.; Cova, E.; Tritto, S.; Faelli, A.; Calamita, G.; Ventura, U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol. Cell 2005, 97, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Ushiki, T.; Mochizuki, T.; Toda, T.; Kudo, T.; Baba, K.; Ishii, M.; Ito, K.; Ochiai, W.; Sugiyama, K. Effects of magnesium sulphate administration on aquaporin 3 in rat gastrointestinal tract. Biol. Pharm. Bull. 2011, 34, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kuramoto, H.; Kadowaki, M. Downregulation in aquaporin 4 and aquaporin 8 expression of the colon associated with the induction of allergic diarrhea in a mouse model of food allergy. Life Sci. 2007, 81, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Itoh, A.; Fukunaga, T.; Satoh, J.; Yasuoka, T.; Fujiyama, Y. Alteration of aquaporin mRNA expression after small bowel resection in the rat residual ileum and colon. J. Gastroenterol. Hepatol. 2003, 18, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Krejs, G.J. VIPoma syndrome. Am. J. Med. 1987, 82, 37–48. [Google Scholar] [CrossRef]
- Kane, M.G.; O’Dorisio, T.M.; Krejs, G.J. Production of secretory diarrhea by intravenous infusion of vasoactive intestinal polypeptide. N. Engl. J. Med. 1983, 309, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Gaginella, T.S.; Capasso, F. The osmotic and intrinsic mechanisms of the pharmacological laxative action of oral high doses of magnesium sulphate. Importance of the release of digestive polypeptides and nitric oxide. Magnes Res. 1996, 9, 133–138. [Google Scholar] [PubMed]
- Ikarashi, N.; Mochiduki, T.; Takasaki, A.; Ushiki, T.; Baba, K.; Ishii, M.; Kudo, T.; Ito, K.; Toda, T.; Ochiai, W.; et al. A mechanism by which the osmotic laxative magnesium sulphate increases the intestinal aquaporin 3 expression in HT-29 cells. Life Sci. 2011, 88, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Riemann, J.F.; Schmidt, H.; Zimmermann, W. The fine structure of colonic submucosal nerves in patients with chronic laxative abuse. Scand. J. Gastroenterol. 1980, 15, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Gaginella, T.S.; Mascolo, N.; Izzo, A.A.; Autore, G.; Capasso, F. Nitric oxide as a mediator of bisacodyl and phenolphthalein laxative action: Induction of nitric oxide synthase. J. Pharmacol. Exp. Ther. 1994, 270, 1239–1245. [Google Scholar] [PubMed]
- Ikarashi, N.; Baba, K.; Ushiki, T.; Kon, R.; Mimura, A.; Toda, T.; Ishii, M.; Ochiai, W.; Sugiyama, K. The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G887–G895. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Gu, Y.; Ishibashi, K.; Marumo, F.; Sasaki, S. Mercury-sensitive residues and pore site in AQP3 water channel. Biochemistry 1997, 36, 13973–13978. [Google Scholar] [CrossRef] [PubMed]
- Zelenina, M.; Tritto, S.; Bondar, A.A.; Zelenin, S.; Aperia, A. Copper inhibits the water and glycerol permeability of aquaporin-3. J. Biol. Chem. 2004, 279, 51939–51943. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Kon, R.; Iizasa, T.; Suzuki, N.; Hiruma, R.; Suenaga, K.; Toda, T.; Ishii, M.; Hoshino, M.; Ochiai, W.; et al. Inhibition of aquaporin-3 water channel in the colon induces diarrhea. Biol. Pharm. Bull. 2012, 35, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Mengs, U.; Rudolph, R.L. Light and electron-microscopic changes in the colon of the guinea pig after treatment with anthranoid and non-anthranoid laxatives. Pharmacology 1993, 47, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Kita, M.; Torihashi, S.; Miyamoto, S.; Won, K.J.; Sato, K.; Ozaki, H.; Karaki, H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G930–G938. [Google Scholar] [PubMed]
- Lee, J.Y.; Cho, B.J.; Park, T.W.; Park, B.E.; Kim, S.J.; Sim, S.S.; Kim, C.J. Dibenzylbutyrolactone lignans from Forsythia koreana fruits attenuate lipopolysaccharide-induced inducible nitric oxide synthetase and cyclooxygenase-2 expressions through activation of nuclear factor-kappab and mitogen-activated protein kinase in RAW264.7 cells. Biol. Pharm. Bull. 2010, 33, 1847–1853. [Google Scholar] [PubMed]
- Lehmann, G.L.; Carreras, F.I.; Soria, L.R.; Gradilone, S.A.; Marinelli, R.A. LPS induces the TNF-alpha-mediated downregulation of rat liver aquaporin-8: Role in sepsis-associated cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G567–G575. [Google Scholar] [CrossRef] [PubMed]
- Horie, I.; Maeda, M.; Yokoyama, S.; Hisatsune, A.; Katsuki, H.; Miyata, T.; Isohama, Y. Tumor necrosis factor-alpha decreases aquaporin-3 expression in DJM-1 keratinocytes. Biochem. Biophys. Res. Commun. 2009, 387, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Purwanti, N.; Karabasil, M.R.; Azlina, A.; Javkhlan, P.; Hasegawa, T.; Akamatsu, T.; Hosoi, T.; Ozawa, K.; Hosoi, K. Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-kappaB and p-c-Jun/c-Fos. Am. J. Pathol. 2010, 177, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zelenina, M.; Christensen, B.M.; Palmer, J.; Nairn, A.C.; Nielsen, S.; Aperia, A. Prostaglandin E(2) interaction with AVP: Effects on AQP2 phosphorylation and distribution. Am. J. Physiol. Ren. Physiol. 2000, 278, F388–F394. [Google Scholar]
- Nejsum, L.N.; Zelenina, M.; Aperia, A.; Frokiaer, J.; Nielsen, S. Bidirectional regulation of AQP2 trafficking and recycling: Involvement of AQP2-S256 phosphorylation. Am. J. Physiol. Ren. Physiol. 2005, 288, F930–F938. [Google Scholar] [CrossRef] [PubMed]
- Kon, R.; Ikarashi, N.; Nagoya, C.; Takayama, T.; Kusunoki, Y.; Ishii, M.; Ueda, H.; Ochiai, W.; Machida, Y.; Sugita, K.; et al. Rheinanthrone, a metabolite of sennoside A, triggers macrophage activation to decrease aquaporin-3 expression in the colon, causing the laxative effect of rhubarb extract. J. Ethnopharmacol. 2014, 152, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Yuan, W.T. Expression of aquaporin 3, 4, and 8 in colonic mucosa of rat models with slow transit constipation. Chin. J. Gastrointest. Surg. 2011, 14, 459–461. [Google Scholar]
- Wang, X.J.; Yuan, W.T.; Song, J.M.; Zhang, Z.Y. Expression and significance of aquaporin 4 in the colonic mucosa of patients with slow transit constipation. Chin. J. Gastrointest. Surg. 2010, 13, 445–447. [Google Scholar]
- Manara, L.; Bianchi, G.; Ferretti, P.; Tavani, A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J. Pharmacol. Exp. Ther. 1986, 237, 945–949. [Google Scholar] [PubMed]
- Kon, R.; Ikarashi, N.; Hayakawa, A.; Haga, Y.; Fueki, A.; Kusunoki, Y.; Tajima, M.; Ochiai, W.; Machida, Y.; Sugiyama, K. Morphine-Induced Constipation Develops With Increased Aquaporin-3 Expression in the Colon via Increased Serotonin Secretion. Toxicol. Sci. 2015, 145, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.F.; Long, J.P. Release of intestinal 5-hydroxytryptamine by morphine and related agents. J. Pharmacol. Exp. Ther. 1967, 156, 267–276. [Google Scholar] [PubMed]
- Kim, M.; Javed, N.H.; Yu, J.G.; Christofi, F.; Cooke, H.J. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J. Clin. Investig. 2001, 108, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L. Neurotransmitter transporters: Fruitful targets for CNS drug discovery. Mol. Psychiatry 2000, 5, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Waku, T.; Shiraki, T.; Oyama, T.; Maebara, K.; Nakamori, R.; Morikawa, K. The nuclear receptor PPARgamma individually responds to serotonin- and fatty acid-metabolites. EMBO J. 2010, 29, 3395–3407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Kim, P.; Lu, Y.F.; Feingold, K.R. PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp. Dermatol. 2011, 20, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Mimura, A.; Kon, R.; Iizasa, T.; Omodaka, M.; Nagoya, C.; Ishii, M.; Toda, T.; Ochiai, W.; Sugiyama, K. The concomitant use of an osmotic laxative, magnesium sulphate, and a stimulant laxative, bisacodyl, does not enhance the laxative effect. Eur. J. Pharm. Sci. 2012, 45, 73–78. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int. J. Mol. Sci. 2016, 17, 1172. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071172
Ikarashi N, Kon R, Sugiyama K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. International Journal of Molecular Sciences. 2016; 17(7):1172. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071172
Chicago/Turabian StyleIkarashi, Nobutomo, Risako Kon, and Kiyoshi Sugiyama. 2016. "Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation" International Journal of Molecular Sciences 17, no. 7: 1172. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071172