Psychopathological Variables and Sleep Quality in Psoriatic Patients
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of the Patients
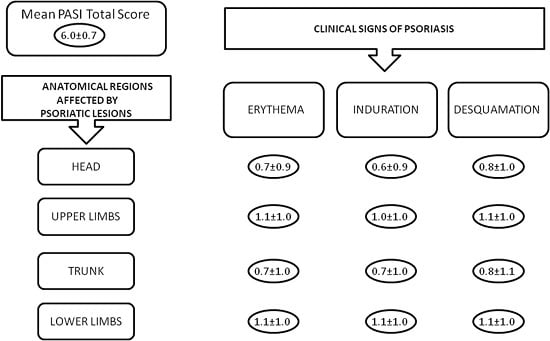
2.2. Dermatological Characteristics of the Patients
2.3. Psychiatric Characteristics of the Patients
2.4. Sleep Quality among the Patients
2.5. Pruritus and Sleep Quality
2.6. Gender Differences
2.7. Pearson’s Correlations
2.8. Unconditional Logistic Regression
3. Discussion
4. Experimental Section
4.1. Materials and Methods
- Zung Self-rating Depression Scale (SDS): a 20-item self-administered questionnaire investigating the presence of depression-related affective, psychological, and somatic symptoms. Each item is scored from 1 to 4, and the subject must report how often they felt or behaved in a certain way (10 questions have a negative connotation and the others have a positive connotation), choosing from the following replies: “a little of the time”, “some of the time”, “good part of the time”, “most of the time”. The total score of the test is then converted into a z score. The questionnaire is reliable in terms of convergent validity and diagnostic discrimination [34,35]. A z score > 50 has been considered as a cut-off for depression.
- Pittsburgh Sleep Quality Index (PSQI): a 19-item self-report questionnaire assessing sleep quality (e.g., sleep latency, sleep disturbances, use of sleeping medication, daytime dysfunction) over a 1-month time interval. In addition, it is possible for the subject to report other conditions, not already mentioned in the PSQI, disturbing their sleep (open answer). Each item is scored on a 0–3 interval scale [36]. Different studies have set different cut-off scores for specific populations [37]. Since higher scores indicate poorer sleep quality, scores above the median value of the sample have been considered as a cut-off for poor sleep quality. The percentage of patients scoring ≥5 has been also reported, since this cut-off has been found to be useful in identifying poor sleepers in most studies [37].
- Interaction Anxiousness Scale (IAS): a 15-item self-rating scale assessing the level of distress when meeting and talking with other people. Some items have a positive connotation. Each item is scored on a five-point scale and the subject must answer to every statement choosing from the following replies: “not at all characteristic of me”, “slightly characteristic of me”, “moderately characteristic of me”, “very characteristic of me”, “extremely characteristic of me”. Higher scores relate to higher levels of interaction anxiety. The IAS has a good validity and internal consistency [38,39]. The scores above the median value of the sample have been considered as a cut-off for interaction anxiety.
- Audience Anxiousness Scale (AAS): a 12-item self-report survey assessing the level of audience anxiety in case of a public performance, from speaking in front of other people to “stage fright”. Some items have a positive connotation. Each item is scored on a five-point scale and the subject must answer to every statement choosing from the following replies: “not at all characteristic of me”, “slightly characteristic of me”, “moderately characteristic of me”, “very characteristic of me”, “extremely characteristic of me”. Higher scores relate to higher levels of audience anxiety. The AAS has a good validity and internal consistency [38]. Scores higher than the median value of the sample have been considered as a cut-off for audience anxiety.
- 0 = 0% of involved area;
- 1 = 1%–9% of involved area;
- 2 = 10%–29% of involved area;
- 3 = 30%–49% of involved area;
- 4 = 50%–69% of involved area;
- 5 = 70%–89% of involved area;
- 6 = 90%–100% of involved area.
4.2. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Krueger, G.G.; Griffiths, C.E. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii18–ii23; discussion ii24–ii25. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, E.A.; Dhillon, J.S.; Armstrong, A.W. Economic burden of psoriasis in the United States: A Systematic Review. JAMA Dermatol. 2015, 151, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, M.J.; Kulkarni, A.; Feldman, S.R.; Balkrishnan, R. Quality of life in patients with psoriasis. Health Qual. Life Outcomes 2006, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Zhao, Y.; Shi, L.; Tran, M.H. Economic and comorbidity burden among patients with moderate-to-severe Psoriasis. J. Manag. Care Spec. Pharm. 2015, 21, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Eder, L.; Haddad, A.; Rosen, C.F.; Lee, K.A.; Chandran, V.; Cook, R.; Gladman, D.D. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: A prospective cohort study. Arthritis Rheumatol. 2016, 68, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Paul, H.K.; Zakaria, S.M.; Islam, M.M.; Shafiquzzaman, M. Epidemiological determinants of psoriasis. Mymensingh Med. J. 2011, 20, 9–15. [Google Scholar] [PubMed]
- De Brouwer, S.J.; van Middendorp, H.; Stormink, C.; Kraaimaat, F.W.; Sweep, F.C.; de Jong, E.M.; Schalkwijk, J.; Eijsbouts, A.; Donders, A.R.; van de Kerkhof, P.C.; et al. The psychophysiological stress response in psoriasis and rheumatoidarthritis. Br. J. Dermatol. 2014, 170, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Jacobson, C.; Weiss, S.; Vreeland, M.G.; Wu, Y. The psychosocial burden of psoriasis. Am. J. Clin. Dermatol. 2005, 6, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hrehorów, E.; Salomon, J.; Matusiak, L.; Reich, A.; Szepietowski, J.C. Patients with psoriasis feel stigmatized. Acta Derm. Venereol. 2012, 92, 67–72. [Google Scholar] [PubMed]
- Sampogna, F.; Tabolli, S.; Abeni, D.; IDI Multipurpose psoriasis research on vital experiences (IMPROVE) investigators. Living with psoriasis: Prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm. Venereol. 2012, 92, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Lakshmy, S.; Balasundaram, S.; Sarkar, S.; Audhya, M.; Subramaniam, E. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J. Psychol. Med. 2015, 37, 434–440. [Google Scholar] [PubMed]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar] [PubMed]
- Cohen, B.E.; Martires, K.J.; Ho, R.S. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009–2012. JAMA Dermatol. 2016, 152, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Malakouti, M.; Koo, J.Y. Social impact of the burden of psoriasis: Effects on patients and practice. Dermatol. Online J. 2014, 20. doj_23523. [Google Scholar]
- Schneider, G.; Heuft, G.; Hockmann, J. Determinants of social anxiety and social avoidance in psoriasis outpatients. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Simpson, F.C.; Gupta, A.K. Psoriasis and sleep disorders: A systematic review. Sleep Med. Rev. 2015, 29, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Güler, S.; Tekatas, A.; Arican, O.; Kaplan, O.S.; Dogru, Y. Restless legs syndrome and insomnia frequency in patients with psoriasis. Ideggyogy. Sz. 2015, 68, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Janowski, K.; Pietrzak, A. Indications for psychological intervention in patients with psoriasis. Dermatol. Ther. 2008, 21, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Golpour, M.; Hosseini, S.H.; Khademloo, M.; Ghasemi, M.; Ebadi, A.; Koohkan, F.; Shahmohammadi, S. Depression and anxiety disorders among patients with psoriasis: A hospital-based case-control study. Dermatol. Res. Pract. 2012, 2012, 381905. [Google Scholar] [CrossRef] [PubMed]
- Remröd, C.; Sjöström, K.; Svensson, Å. Subjective stress reactivity in psoriasis—A cross sectional study of associated psychological traits. BMC Dermatol. 2015, 15, 6. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K.; Kirkby, S.; Schork, N.J.; Gorr, S.K.; Ellis, C.N.; Voorhees, J.J. A psychocutaneous profile of psoriasis patients who are stress reactors. A study of 127 patients. Gen. Hosp. Psychiatry 1989, 11, 166–173. [Google Scholar] [CrossRef]
- Zachariae, R.; Zachariae, H.; Blomqvist, K.; Davidsson, S.; Molin, L.; Mørk, C.; Sigurgeirsson, B. Self-reported stress reactivity and psoriasis-related stress of Nordic psoriasis sufferers. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Rabin, F.; Bhuiyan, S.I.; Islam, T.; Haque, M.A.; Islam, M.A. Psychiatric and psychological comorbidities in patients with psoriasis—A review. Mymensingh Med. J. 2012, 21, 780–786. [Google Scholar] [PubMed]
- Ryan, C.; Sadlier, M.; de Vol, E.; Patel, M.; Lloyd, A.A.; Day, A.; Lally, A.; Kirby, B.; Menter, A. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J. Am. Acad. Dermatol. 2015, 72, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Nasca, M.R.; Micali, G. Videodermatoscopy enhances diagnostic capability in psoriatic balanitis. J. Am. Acad. Dermatol. 2009, 61, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Halioua, B.; Sid-Mohand, D.; Roussel, M.E.; Maury-le-Breton, A.; de Fontaubert, A.; Stalder, J.F. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Shim, W.H.; Kim, J.M.; Mun, J.H.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, M.B.; Kim, B.S. Clinical characteristics of pruritus in patients with scalp psoriasis and their relation with intraepidermal nerve fiber density. Ann. Dermatol. 2014, 26, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Nakamura, Y.; Aoki, R.; Ishimaru, K.; Ogawa, H.; Okumura, K.; Shibata, S.; Shimada, S.; Nakao, A. Circadian gene Clock regulates psoriasis-like skin inflammation in mice. J. Investig. Dermatol. 2015, 135, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Stinco, G.; Trevisan, G.; Piccirillo, F.; di Meo, N.; Nan, K.; Deroma, L.; Bergamo, S.; Patrone, P. Psoriasis vulgaris does not adversely influence the quality of sleep. G Ital. Dermatol. Venereol. 2013, 148, 655–659. [Google Scholar] [PubMed]
- Lavery, M.J.; Stull, C.; Kinney, M.O.; Yosipovitch, G. Nocturnal pruritus: The battle for a peaceful night’s sleep. Int. J. Mol. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Szepietowski, J.C. Mediators of pruritus in psoriasis. Mediat. Inflamm. 2007, 2007, 64727. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C.; Rydlewski, M.; Araújo, M.S.; Tufik, S.; Andersen, M.L. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS ONE 2012, 7, e51183. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.K. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Thurber, S.; Snow, M.; Honts, C.R. The Zung self-rating depression scale: Convergent validity and diagnostic discrimination. Assessment 2002, 9, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Leary, M.R. Social anxiousness: The construct and its measurement. J. Pers. Assess. 1983, 47, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Leary, M.R.; Kowalski, R.M. The interaction anxiousness Scale: Construct and criterion-related validity. J. Pers. Assess. 1993, 61, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, T.; Pettersson, U. Severe psoriasis—Oral therapy with a new retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wozel, G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005, 210, 194–199. [Google Scholar] [CrossRef] [PubMed]

| Variables | Values |
|---|---|
| Age | 48.7 ± 13.6 |
| Female | 49, 48% |
| Male | 53, 52% |
| Ethnicity: Caucasian | 101, 99% |
| Ethnicity: Indian | 1, 1% |
| BMI | 27.3 ± 5.3 |
| Years of education | 10.6 ± 3.8 |
| Comorbidity | |
| Hypertension | 30, 29.4% |
| Diabetes | 10, 9.8% |
| Anxiety | 5, 4.9% |
| Working characteristics | |
| Unemployed | 13, 12.7% |
| Employed | 59, 57.8% |
| Housewife/retired | 30, 29.4% |
| PASI Score | OR | 95% CI | p-Value |
|---|---|---|---|
| Head | |||
| Erythema | 1.9 | 0.74–4.99 | 0.175 |
| Induration | 0.8 | 0.34–2.05 | 0.705 |
| Desquamation | 0.7 | 0.30–1.68 | 0.441 |
| Upper limbs | |||
| Erythema | 1.0 | 0.54–1.96 | 0.921 |
| Induration | 0.95 | 0.42–2.16 | 0.915 |
| Desquamation | 1.2 | 0.54–2.60 | 0.657 |
| Trunk | |||
| Erythema | 1.3 | 0.52–3.47 | 0.536 |
| Induration | 0.3 | 0.09–1.15 | 0.08 |
| Desquamation | 2.4 | 0.81–7.10 | 0.11 |
| Lower limbs | |||
| Erythema | 2.8 | 1.08–7.2 | 0.033 |
| Induration | 0.5 | 0.19–1.13 | 0.09 |
| Desquamation | 1.5 | 0.68–3.19 | 0.319 |
| PASI Score | OR | 95% CI | p-Value |
|---|---|---|---|
| Head | |||
| Erythema | 14.8 | 1.4–147.2 | 0.021 |
| Induration | 0.6 | 0.23–1.47 | 0.258 |
| Desquamation | 1.1 | 0.46–2.67 | 0.805 |
| Upper limbs | |||
| Erythema | 1.6 | 0.81–3.33 | 0.163 |
| Induration | 0.9 | 0.37–2.07 | 0.777 |
| Desquamation | 0.4 | 0.21–1.11 | 0.08 |
| Trunk | |||
| Erythema | 2.0 | 0.73–5.42 | 0.173 |
| Induration | 0.4 | 0.13–1.54 | 0.204 |
| Desquamation | 1.2 | 0.44–3.46 | 0.672 |
| Lower limbs | |||
| Erythema | 1.6 | 0.80–3.18 | 0.183 |
| Induration | 0.7 | 0.32–1.71 | 0.498 |
| Desquamation | 0.9 | 0.42–1.87 | 0.758 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, M.; Luca, A.; Musumeci, M.L.; Fiorentini, F.; Micali, G.; Calandra, C. Psychopathological Variables and Sleep Quality in Psoriatic Patients. Int. J. Mol. Sci. 2016, 17, 1184. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071184
Luca M, Luca A, Musumeci ML, Fiorentini F, Micali G, Calandra C. Psychopathological Variables and Sleep Quality in Psoriatic Patients. International Journal of Molecular Sciences. 2016; 17(7):1184. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071184
Chicago/Turabian StyleLuca, Maria, Antonina Luca, Maria Letizia Musumeci, Federica Fiorentini, Giuseppe Micali, and Carmela Calandra. 2016. "Psychopathological Variables and Sleep Quality in Psoriatic Patients" International Journal of Molecular Sciences 17, no. 7: 1184. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071184