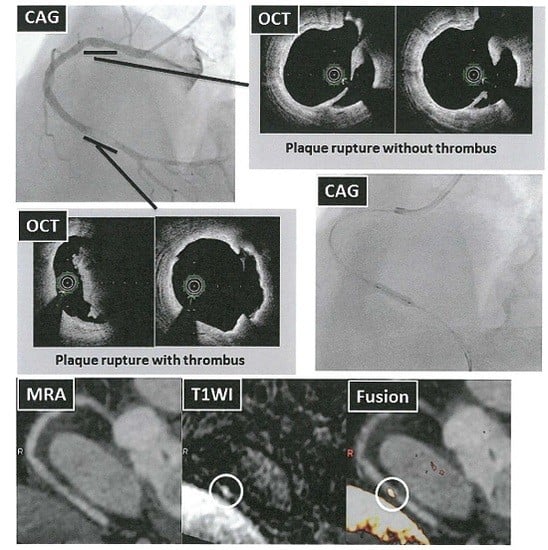
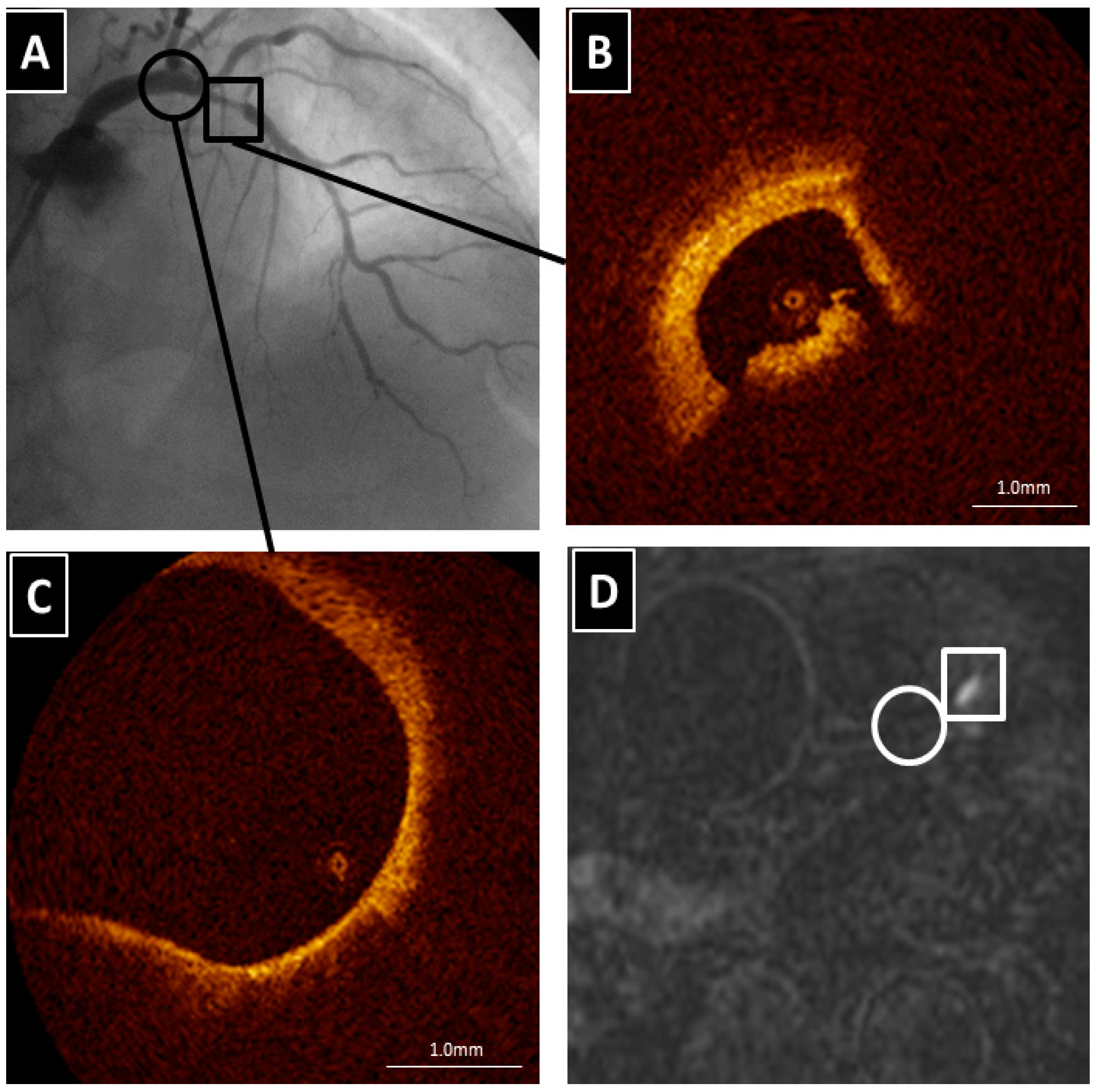
The Clinical Value of High-Intensity Signals on the Coronary Atherosclerotic Plaques: Noncontrast T1-Weighted Magnetic Resonance Imaging
Abstract
:1. Introduction
2. The Beginning of Coronary Artery Plaque Imaging Based on High-Intensity Signal on Noncontrast T1-Weighted Imaging
3. What Appears as High-intensity Signal on T1-Weighted Imaging in the Coronary Artery? What Is the Best PMR Cutoff Value?
4. The Clinical Implication of High-Intensity Signal in Coronary Atherosclerotic Plaques
5. Limitations and Issues to Be Resolved in the Future
6. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| TCFA | thin-cap fibroatheroma |
| IVUS | intravascular ultrasound |
| OCT | optical coherence tomography |
| MR | magnetic resonance |
| CT | computed tomography |
| T1WI | T1-weighted imaging |
| HIS | high-intensity signal |
| PMR | the ratio between the signal intensities of coronary plaque and cardiac muscle |
| PCI | percutaneous coronary intervention |
| FNR | filter no-reflow |
References
- Braunwald, E. Acute myocardial infarction—The value of being prepared. N. Engl. J. Med. 1996, 334, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; dal Bello, B.; Morbini, P.; Burke, A.P.; Bocciarelli, M.; Specchia, G.; Virmani, R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999, 82, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Schoenhagen, P.; Ziada, K.M.; Kapadia, S.R.; Crowe, T.D.; Nissen, SE.; Tuzcu, E.M. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: An intravascular ultrasound study. Circulation 2000, 101, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Ehara, S.; Kobayashi, Y.; Yoshiyama, M.; Shimada, K.; Shimada, Y.; Fukuda, D.; Nakamura, Y.; Yamashita, H.; Yamagishi, H.; Takeuchi, K.; et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation 2004, 110, 3424–3429. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Carlier, S.G.; Mintz, G.S.; Takebayashi, H.; Yasuda, T.; Costa, R.A.; Moussa, I.; Dangas, G.; Meran, R.; Lansky, A.J.; et al. Intravascular ultrasound study of patterns of calcium in ruptured coronary plaques. Am. J. Cardiol. 2005, 96, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu, K.; Takano, M.; Sakai, S.; Ishibashi, F.; Uemura, R.; Takano, T.; Mizuno, K. Elevated troponin T levels and lesion characteristics in non-ST-elevation acute coronary syndromes. Circulation 2004, 109, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, T.; Ueda, Y.; Mizote, I.; Oyabu, J.; Okada, K.; Hirayama, A.; Kodama, K. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: Detection of vulnerable patients by angioscopy. J. Am. Coll. Cardiol. 2006, 47, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of culprit lesion morphology in acute myocardial infarction: Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, M.; Imanishi, T.; Tanaka, A.; Kubo, T.; Liu, Y.; Takarada, S.; Kitabata, H.; Tanimoto, T.; Komukai, K.; Ishibashi, K.; et al. Clinical classification and plaque morphology determined by optical coherence tomography in unstable angina pectoris. Am. J. Cardiol. 2010, 106, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, H.; Bourantas, C.; Bagnall, A.; Mintz, G.S.; Kunadian, V. OCT for the identification of vulnerable plaque in acute coronary syndrome. J. Am. Coll. Cardiol. 2015, 8, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, T.S.; Ross, R.; Polissar, N.L.; Yuan, C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000, 102, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Mitsumori, L.M.; Beach, K.W.; Maravilla, K.R. Carotid atherosclerotic plaque: Noninvasive MR characterization and identification of vulnerable lesions. Radiology 2001, 221, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Moody, A.R.; Murphy, R.E.; Morgan, P.S.; Martel, A.L.; Delay, G.S.; Allder, S.; MacSweeney, S.T.; Tennant, W.G.; Gladman, J.; Lowe, J.; et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003, 107, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.E.; Moody, A.R.; Morgan, P.S.; Martel, A.L.; Delay, G.S.; Allder, S.; MacSweeney, S.T.; Tennant, W.G.; Gladman, J.; Lowe, J.; et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation 2003, 107, 3053–3058. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Hunt, B.J.; Moody, A. Magnetic resonance direct thrombus imaging: A novel technique for imaging venous thromboemboli. Thromb. Haemost. 2003, 89, 773–782. [Google Scholar] [PubMed]
- Fayad, Z.A.; Fuster, V.; Fallon, J.T.; Jayasundera, T.; Worthley, S.G.; Helft, G.; Aguinaldo, J.G.; Badimon, J.J.; Sharma, S.K. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 2000, 102, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Nguyen, P.K.; Rubin, G.D.; Meyer, C.H.; Shimakawa, A.; Nishimura, D.G.; Ehara, S.; Iribarren, C.; Courtney, B.K.; Go, A.S.; et al. Right coronary wall CMR in the older asymptomatic advance cohort: positive remodeling and associations with type 2 diabetes and coronary calcium. J. Cardiovasc. Magn. Reson. 2010, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Koga, S.; Koga, N.; Noguchi, T.; Tanaka, H.; Koga, H.; Serikawa, T.; Orita, Y.; Ikeda, S.; Mito, T.; et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: Comparison with multislice computed tomography and intravascular ultrasound. J. Am. Coll. Cardiol. 2009, 2, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.H.; Perera, D.; Makowski, M.R.; Wiethoff, A.J.; Phinikaridou, A.; Razavi, R.M.; Marber, M.S.; Greil, G.F.; Nagel, E.; Maintz, D.; et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 2011, 124, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Ehara, S.; Hasegawa, T.; Nakata, S.; Matsumoto, K.; Nishimura, S.; Iguchi, T.; Kataoka, T.; Yoshikawa, J.; Yoshiyama, M. Hyperintense plaque identified by magnetic resonance imaging relates to intracoronary thrombus as detected by optical coherence tomography in patients with angina pectoris. Eur. Heart J. 2012, 13, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Kawasaki, T.; Tanaka, A.; Yasuda, S.; Goto, Y.; Ishihara, M.; Nishimura, K.; Miyamoto, Y.; Node, K.; Koga, N. High-intensity signals in coronary plaques on non-contrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J. Am. Coll. Cardiol. 2014, 63, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ehara, S.; Hasegawa, T.; Sakaguchi, M.; Otsuka, K.; Yoshikawa, J.; Shimada, K. Localization of coronary high-intensity signals on T1-weighted MR imaging: Relation to plaque morphology and clinical severity of angina pectoris. J. Am. Coll. Cardiol. 2015, 8, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Quick, H.H.; Debatin, J.F.; Ladd, M.E. MR imaging of the vessel wall. Eur. Radiol. 2002, 12, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Imanishi, T.; Kashiwagi, M.; Ikejima, H.; Tsujioka, H.; Kuroi, A.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; Ino, Y.; et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am. J. Cardiol. 2010, 105, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Asaumi, Y.; Noguchi, T.; Morita, Y.; Fujiwara, R.; Kanaya, T.; Matsuyama, TA.; Kawasaki, T.; Fujino, M.; Yamane, T.; Nagai, T.; et al. High-intensity plaques on noncontrast T1-weighted imaging as a predictor of periprocedural myocardial injury. J. Am. Coll. Cardiol. 2015, 8, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Sato, A.; Akiyama, D.; Hiraya, D.; Sakai, S.; Shindo, M.; Mori, K.; Minami, M.; Aonuma, K. Coronary high-intensity plaque on T1-weighted magnetic resonance imaging and its association with myocardial injury after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ehara, S.; Hasegawa, T.; Otsuka, K.; Yoshikawa, J.; Shimada, K. Prediction of the filter no-reflow phenomenon in patients with angina pectoris by using multimodality: Magnetic resonance imaging, optical coherence tomography, and serum biomarkers. J. Cardiol. 2016, 67, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S. The “no reflow” phenomenon following acute myocardial infarction: Mechanisms and treatment options. J. Cardiol. 2014, 64, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Mol, G.C.; van Rooden, C.J.; Klok, F.A.; Westerbeek, R.E.; del Sol, A.I.; van de Ree, M.A.; de Roos, A.; Huisman, M.V. Magnetic resonance direct thrombus imaging differentiates acute recurrent ipsilateral deep vein thrombosis from residual thrombosis. Blood 2014, 124, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Kampschulte, A.; Ferguson, M.S.; Kerwin, W.S.; Yarnykh, V.L.; O’Brien, K.D.; Polissar, N.L.; Hatsukami, T.S.; Yuan, C. Hemorrhage in the atherosclerotic carotid plaque: A high-resolution MRI study. Stroke 2004, 35, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Underhill, H.R.; Hippe, D.S.; Xue, Y.; Yuan, C.; Hatsukami, T.S. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: A long-term time course study. J. Am. Coll. Cardiol. 2012, 5, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Tanaka, A.; Kawasaki, T.; Goto, Y.; Morita, Y.; Asaumi, Y.; Nakao, K.; Fujiwara, R.; Nishimura, K.; Miyamoto, Y.; et al. Effect of intensive statin therapy on coronary high-intensity plaques detected by noncontrast T1-weighted imaging. J. Am. Coll. Cardiol. 2015, 66, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Briley-Saebo, K.C.; Shaw, P.X.; Mulder, W.J.; Choi, S.H.; Vucic, E.; Aguinaldo, J.G.; Witztum, J.L.; Fuster, V.; Tsimikas, S.; Fayad, Z.A. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation 2008, 117, 3206–3215. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehara, S.; Matsumoto, K.; Shimada, K. The Clinical Value of High-Intensity Signals on the Coronary Atherosclerotic Plaques: Noncontrast T1-Weighted Magnetic Resonance Imaging. Int. J. Mol. Sci. 2016, 17, 1187. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071187
Ehara S, Matsumoto K, Shimada K. The Clinical Value of High-Intensity Signals on the Coronary Atherosclerotic Plaques: Noncontrast T1-Weighted Magnetic Resonance Imaging. International Journal of Molecular Sciences. 2016; 17(7):1187. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071187
Chicago/Turabian StyleEhara, Shoichi, Kenji Matsumoto, and Kenei Shimada. 2016. "The Clinical Value of High-Intensity Signals on the Coronary Atherosclerotic Plaques: Noncontrast T1-Weighted Magnetic Resonance Imaging" International Journal of Molecular Sciences 17, no. 7: 1187. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071187