Design of Phosphonated Imidazolium-Based Ionic Liquids Grafted on γ-Alumina: Potential Model for Hybrid Membranes
Abstract
:1. Introduction
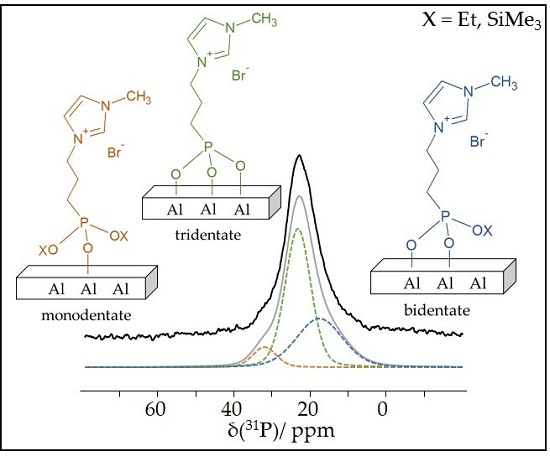
2. Results and Discussion
3. Materials and Methods
3.1. Starting Materials
3.2. Grafting Reactions
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of Ionic Liquids with Membrane Technology: A New Approach for CO2 Separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Nair, J.R.; Porcarelli, L.; Bella, F.; Gerbaldi, C. Newly Elaborated Multipurpose Polymer Electrolyte Encompassing RTILs for Smart Energy-Efficient Devices. ACS Appl. Mater. Interfaces 2015, 7, 12961–12971. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, Y.S.; Kim, Y.; Hong, W.H.; Song, H. 1D and 3D Ionic Liquid–Aluminum Hydroxide Hybrids Preparedvia an Ionothermal Process. Adv. Funct. Mater. 2007, 17, 2411–2418. [Google Scholar] [CrossRef]
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super Soft All-Ethylene Oxide Polymer Electrolyte for Safe All-Solid Lithium Batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef] [PubMed]
- Zehbe, K.; Kollosche, M.; Lardong, S.; Kelling, A.; Schilde, U.; Taubert, A. Ionogels Based on Poly(methyl methacrylate) and Metal-Containing Ionic Liquids: Correlation between Structure and Mechanical and Electrical Properties. Int. J. Mol. Sci. 2016, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Scovazzo, P.; Kieft, J.; Finan, D.A.; Koval, C.; Dubois, D.; Noble, R. Gas separations using non-hexafluorophosphate [PF6]− anion supported ionic liquid membranes. J. Membr. Sci. 2004, 238, 57–63. [Google Scholar] [CrossRef]
- Albo, J.; Yoshioka, T.; Tsuru, T. Porous Al2O3/TiO2 tubes in combination with 1-ethyl-3-methylimidazolium acetate ionic liquid for CO2/N2 separation. Sep. Purif. Technol 2014, 122, 440–448. [Google Scholar] [CrossRef]
- Mehnert, C.P. Supported ionic liquid catalysis. Chem. Eur. J. 2004, 11, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, C.; Wahlen, J.; Mertens, P.; Binnemans, K.; de Vos, D. Immobilization of molecular catalysts in supported ionic liquid phases. Dalton Trans. 2010, 39, 8377–8390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Takafuji, M.; Liu, X.; Jiang, S.; Ihara, H. Investigation of π–π and ion-dipole interactions on 1-allyl-3-butylimidazolium ionic liquid-modified silica stationary phase in reversed-phase liquid chromatography. J. Chromatogr. A 2010, 1217, 5190–5196. [Google Scholar] [CrossRef] [PubMed]
- Pino, V.; Afonso, A.M. Surface-bonded ionic liquid stationary phases in high-performance liquid chromatography-A review. Anal. Chim. Acta 2012, 714, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, M.; Bi, W.; Row, K.H. Application of Ionic Liquids in High Performance Reversed-Phase Chromatography. Int. J. Mol. Sci. 2009, 10, 2591–2610. [Google Scholar] [CrossRef] [PubMed]
- Perdikaki, A.V.; Vangeli, O.C.; Karanikolos, G.N.; Stefanopoulos, K.L.; Beltsios, K.G.; Alexandridis, P.; Kanellopoulos, N.K.; Romanos, G.E. Ionic Liquid-Modified Porous Materials for Gas Separation and Heterogeneous Catalysis. J. Phys. Chem. C 2012, 116, 16398–16411. [Google Scholar] [CrossRef]
- Vangeli, O.C.; Romanos, G.E.; Beltsios, K.G.; Fokas, D.; Kouvelos, E.P.; Stefanopoulos, K.L.; Kanellopoulos, N.K. Grafting of imidazolium based ionic liquid on the pore surface of nanoporous materials—Study of physicochemical and thermodynamic properties. J. Phys. Chem. B 2010, 114, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Fehrmann, R.; Haumann, M.; Riisager, A. Introduction. In Supported Ionic Liquids: Fundamentals and Applications, 1st ed.; Fehrmann, R., Riisager, A., Haumann, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–9. [Google Scholar]
- Gadenne, B.; Hesemann, P.; Moreau, J.J.E. Supported ionic liquids: Ordered mesoporous silicas containing covalently linked ionic species. Chem. Commun. 2004, 1768–1769. [Google Scholar] [CrossRef] [PubMed]
- Motos-Pérez, B.; Roeser, J.; Thomas, A.; Hesemann, P. Imidazolium functionalized SBA-15 type silica: Efficient organocatalysts for Henry and cycloaddition reactions. Appl. Organomet. Chem. 2013, 27, 290–299. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Hesemann, P.; Moreau, J.J.E. I-Silica: Nanostructured silica hybrid materials containing imidazolium groups by hydrolysis-polycondensation of disilylated bis-N,N′-alkyl-imidazolium halides. Microporous Mesoporous Mater. 2011, 142, 292–300. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wei, Y. A silica gel supported dual acidic ionic liquid: An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Green Chem. 2010, 12, 2246–2254. [Google Scholar] [CrossRef]
- Kotadia, D.A.; Soni, S.S. Silica gel supported-SO3H functionalized benzimidazolium based ionic liquid as a mild and effective catalyst for rapid synthesis of 1-amidoalkyl naphthols. J. Mol. Catal. A Chem. 2012, 353–354, 44–49. [Google Scholar] [CrossRef]
- Zhu, J.M.; Xin, F.; Sun, Y.C.; Dong, X.C. Phosphonium-based ionic liquids grafted onto silica for CO2 sorption. Theor. Found. Chem. Eng. 2014, 48, 787–792. [Google Scholar] [CrossRef]
- Julbe, A.; Farrusseng, D.; Guizard, C. Porous ceramic membranes for catalytic and reactors and overview and new ideas. J. Membr. Sci. 2001, 181, 3–20. [Google Scholar] [CrossRef]
- Xin, B.; Hao, J. Imidazolium-based ionic liquids grafted on solid surfaces. Chem. Soc. Rev. 2014, 43, 7171–7187. [Google Scholar] [CrossRef] [PubMed]
- Alami-Younssi, S.; Kiefer, C.; Larbot, A.; Persin, M.; Sarrazin, J. Grafting γ-alumina microporous membranes by organosilanes: Characterisation by pervaporation. J. Membr. Sci. 1998, 143, 27–36. [Google Scholar] [CrossRef]
- Leger, C.; De Lira, H.L.; Paterson, R. Preparation and properties of surface modified ceramic membranes. Part III. Gas permeation of 5 nm alumina membranes modified by trichloro-octadecylsilane. J. Membr. Sci. 1996, 120, 187–195. [Google Scholar] [CrossRef]
- Randon, J.; Blanc, P.; Paterson, R. Modification of ceramic membrane surfaces using phosphoric acid and alkyl phosphonic acids and its effects on ultrafiltration of BSA protein. J. Membr. Sci. 1995, 98, 119–129. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P. Chemically modified ceramic membranes. Microporous Mesoporous Mater. 1998, 22, 321–332. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Organically modified aluminas by grafting and sol–gel processes involving phosphonate derivatives. J. Mater. Chem. 2001, 11, 3161–3165. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Anchoring of Phosphonate and Phosphinate Coupling Molecules on Titania Particles. Chem. Mater. 2001, 13, 4367–4373. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Almaric, J. Preparation of An Inorganic Substrate Having Antimicrobial Properties. Patent No. US 8586758 B2, 19 November 2013. CNRS (FR); Université Montpellier II (FR). [Google Scholar]
- Mu, Z.; Zhou, F.; Zhang, S.; Liang, Y.; Liu, W. Preparation and Characterization of New Phosphonyl-Substitued Imidazolium ionic Liquids. Helv. Chim. Acta 2004, 87, 2549–2555. [Google Scholar] [CrossRef]
- McKenna, C.; Schmidhauser, J. Functional Selectivity in Phosphonate Ester Dealkylation with Bromotrimethylsilane. J. Chem. Soc. Chem. Commun. 1979, 17, 739. [Google Scholar] [CrossRef]
- Leenaars, A.F.M.; Keizer, K.; Bruggraaf, A.J. The reparation and characterization of alumina membrane with ultra-fine pores. J. Mater. Sci. 1984, 19, 1077–1088. [Google Scholar] [CrossRef]
- Guerrero, G.; Alauzun, J.G.; Granier, M.; Laurencin, D.; Mutin, P.H. Phosphonate coupling molecules for the control of surface/interface properties and the synthesis of nanomaterials. Dalton Trans. 2013, 42, 12569–12585. [Google Scholar] [CrossRef] [PubMed]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Hybrid material from organophosphorus Coupling Molecules. J. Mater. Chem. 2005, 15, 3761–3768. [Google Scholar] [CrossRef]
- Galarneau, A.; Abid, Z.; Said, B.; Didi, Y.; Szymanska, K.; Jarzębski, A.; Tancret, F.; Hamaizi, H.; Bengueddach, A.; Di Renzo, F.; et al. Synthesis and Textural Characterization of Mesoporous and Meso-/Macroporous Silica Monoliths Obtained by Spinodal Decomposition. Inorganics 2016, 4, 9. [Google Scholar] [CrossRef]
- Thissen, P.; Vega, A.; Peixoto, T.; Chabal, J.Y. Environment-Controlled Tethering by Aggregation and Growth of Phosphonic Acid Monolayers on Silicon Oxide. Langmuir 2012, 28, 8046–8051. [Google Scholar]
- Quiñones, R.; mariea, S.; Gawalt, E. Study of the Formation of Self-Assembled Monolayers on Nitinol. Langmuir 2007, 23, 10123–10130. [Google Scholar] [CrossRef] [PubMed]
- Brodard-Severac, F.; Guerrero, G.; Maquet, J.; Florian, P.; Gervais, C.; Mutin, P.H. High-Field 17O MAS NMR Investigation of Phosphonic Acid Monolayers on Titania. Chem. Mater. 2008, 20, 5191–5196. [Google Scholar] [CrossRef]
- Taoufik, M.; Szeto, K.C.; Merle, N.; Del Rosal, I.; Maron, L.; Trébosc, J.; Tricot, G.; Gauvin, R.M.; Delevoye, L. Heteronuclear NMR spectroscopy as a surface-selective technique: A unique look at the hydroxyl groups of γ-alumina. Chem. Eur. J. 2014, 20, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.W.; Newland, G.L. Organophosphorus compounds. P-Arylated perhydro-1,2-azaphosphorines. Aust. J. Chem. 1977, 30, 579. [Google Scholar] [CrossRef]





| IL | Solvent (mL) | T (°C) | Time (h) | N-Fold Excess | N (ILs) mmol | Sample | |
|---|---|---|---|---|---|---|---|
| Standard conditions | ImPE | 2-butanol (5) | 90 | 288 | 6 | 3.6 | ImPE1 |
| ImTMSP | CH2Cl2 (14) | 25 | 17 | 2 | 1.2 | ImTMSP1 | |
| ImTMSP | CH2Cl2 (14) | 25 | 72 | 2 | 1.2 | ImTMSP2 | |
| ImTMSP | CH2Cl2 (14) | 25 | 17 | 6 | 3.6 | ImTMSP3 | |
| ImTMSP | CH2Cl2 (14) | 25 | 72 | 6 | 3.6 | ImTMSP4 | |
| Forcing conditions | ImPE | water (10) | 130 | 17 | 2 | 1.2 | ImPE2 |
| ImPE | water (10) | 130 | 17 | 6 | 3.6 | ImPE3 | |
| ImPE | water (10) | 130 | 17 | 12 | 7.2 | ImPE4 |
| Sample | CBET | wt% P a | P nm−2 b |
|---|---|---|---|
| γ-Al2O3 | 82 | 0 | / |
| ImPE1 | 60 | 0.90 ± 0.05 | 0.9 |
| ImPE2 | 63 | 0.62 ± 0.02 | 0.6 |
| ImPE3 | 59 | 1.12 ± 0.04 | 1.1 |
| ImPE4 | 55 | 1.42 ± 0.03 | 1.4 |
| ImTMSP1 | 70 | 0.92 ± 0.04 | 0.9 |
| ImTMSP2 | 66 | 0.92 ± 0.02 | 0.9 |
| ImTMSP3 | 64 | 1.16 ± 0.10 | 1.1 |
| ImTMSP4 | 63 | 1.00 ± 0.10 | 1.0 |
| Sample | ImPE1 | ImPE2 | ImPE3 | ImPE4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ (ppm) | 31.6 | 22.1 | 18.1 | 32.4 | 23.6 | 17.9 | 32.4 | 23.6 | 17.9 | 32.4 | 23.6 | 17.9 |
| Width (ppm) | 7.5 | 7.6 | 11.5 | 9.1 | 6.9 | 10.0 | 7.4 | 7.4 | 13.0 | 7.4 | 8.0 | 14.4 |
| Integration (%) | 18 | 37 | 45 | 13 | 58 | 29 | 7 | 55 | 38 | 7 | 57 | 36 |
| Sample | ImTMSP1 | ImTMSP2 | ImTMSP3 | ImTMSP4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ (ppm) | 25.6 | 22.1 | 18.2 | 25.6 | 22.1 | 18.2 | 25.6 | 22.1 | 18.2 | 25.6 | 22.1 | 18.2 |
| Width (ppm) | 9.6 | 5.5 | 12 | 9 | 5.8 | 12.6 | 9 | 4.9 | 11.2 | 9 | 4.9 | 10.8 |
| Integration (%) | 18 | 35 | 47 | 9 | 30 | 62 | 10 | 23 | 67 | 13 | 29 | 58 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzoccaro, M.-A.; Drobek, M.; Petit, E.; Guerrero, G.; Hesemann, P.; Julbe, A. Design of Phosphonated Imidazolium-Based Ionic Liquids Grafted on γ-Alumina: Potential Model for Hybrid Membranes. Int. J. Mol. Sci. 2016, 17, 1212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081212
Pizzoccaro M-A, Drobek M, Petit E, Guerrero G, Hesemann P, Julbe A. Design of Phosphonated Imidazolium-Based Ionic Liquids Grafted on γ-Alumina: Potential Model for Hybrid Membranes. International Journal of Molecular Sciences. 2016; 17(8):1212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081212
Chicago/Turabian StylePizzoccaro, Marie-Alix, Martin Drobek, Eddy Petit, Gilles Guerrero, Peter Hesemann, and Anne Julbe. 2016. "Design of Phosphonated Imidazolium-Based Ionic Liquids Grafted on γ-Alumina: Potential Model for Hybrid Membranes" International Journal of Molecular Sciences 17, no. 8: 1212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081212