SMA Human iPSC-Derived Motor Neurons Show Perturbed Differentiation and Reduced miR-335-5p Expression
Abstract
:1. Introduction
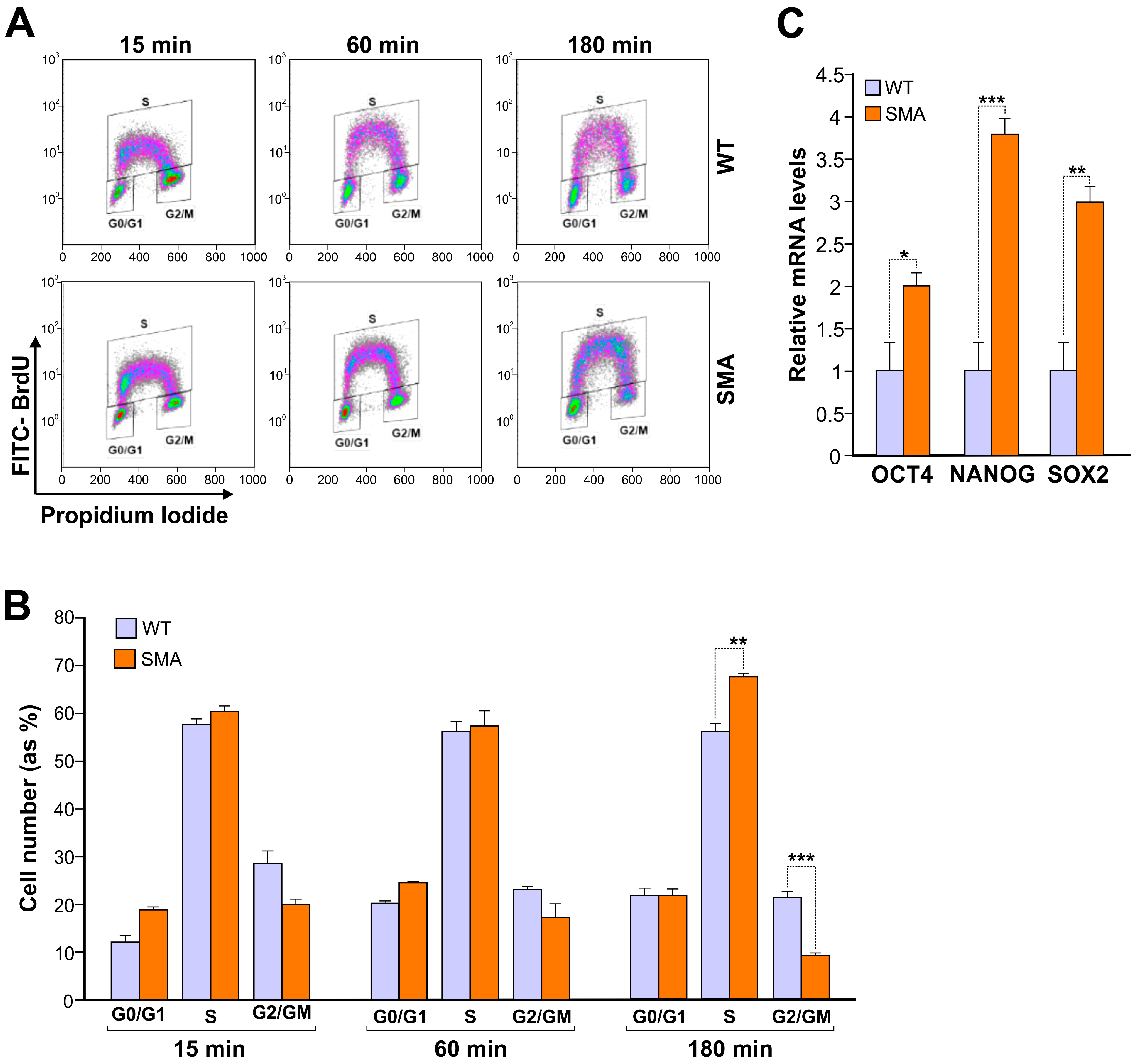
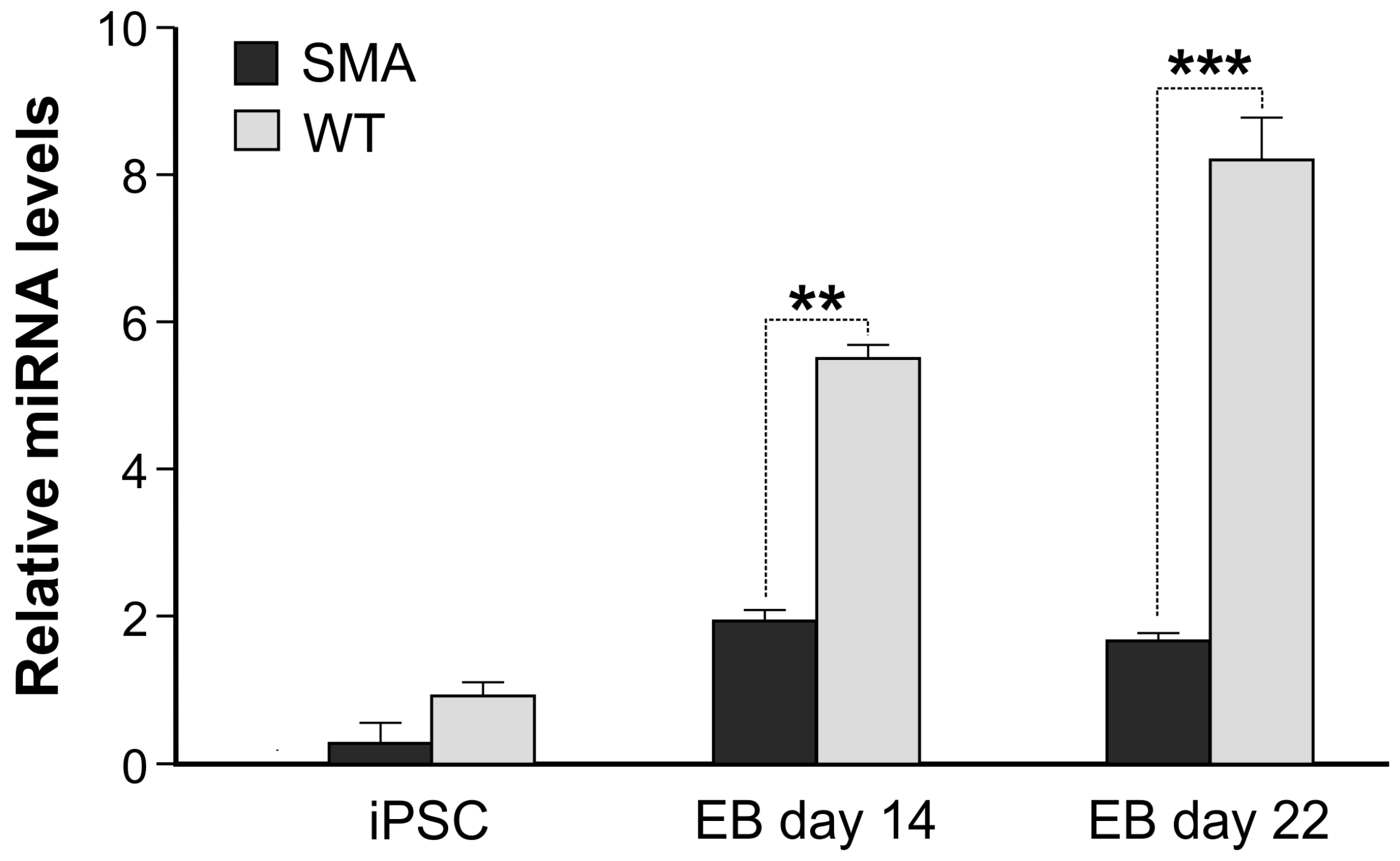
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cell Culture and Reprogramming to hiPSCs
4.3. Cytofluorimetry
4.4. Expression Analyses
4.5. hiPSCs Differentiation and Immunocytochemical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Coovert, D.D.; Le, T.T.; McAndrew, P.E.; Strasswimmer, J.; Crawford, T.O.; Mendell, J.R.; Coulson, S.E.; Androphy, E.J.; Prior, T.W.; Burghes, A.H. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997, 6, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.O.; Pardo, C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996, 3, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Svendsen, C.N. Stem cell model of spinal muscular atrophy. Arch. Neurol. 2010, 67, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Frattini, E.; Ruggieri, M.; Salani, S.; Faravelli, I.; Zanetta, C.; Nizzardo, M.; Simone, C.; Magri, F.; Corti, S. Pluripotent stem cell-based models of spinal muscular atrophy. Mol. Cell. Neurosci. 2015, 64, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Stappert, L.; Roese-Koerner, B.; Brustle, O. The role of microRNAs in human stem cells, neuronal differentiation and subtype specification. Cell Tissue Res. 2015, 359, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Scarola, M.; Comisso, E.; Schneider, C.; Benetti, R. An OCT4-pRb axis, controlled by miR-335, integrates stem cell self-renewal and cell cycle control. Stem Cells 2013, 31, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, A.; Ciafrè, S.A.; Murdocca, M.; Malgieri, A.; Masotti, A.; Sanchez, M.; Farace, M.G.; Novelli, G.; Sangiuolo, F. A perturbed microRNA expression pattern characterizes embryonic neural stem cells derived from a severe mouse model of Spinal Muscular Atrophy (SMA). Int. J. Mol. Sci. 2015, 16, 18312–18327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briscoe, J.; Ericson, J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001, 11, 43–49. [Google Scholar] [CrossRef]
- Arber, S.; Han, B.; Mendelsohn, M.; Smith, M.; Jessell, T.M.; Sockanathan, S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 1999, 23, 659–674. [Google Scholar] [CrossRef]
- Spitalieri, P.; Talarico, R.V.; Botta, A.; Murdocca, M.; D’Apice, M.R.; Orlandi, A.; Giardina, E.; Santoro, M.; Brancati, F.; Novelli, G.; et al. Generation of human induced pluripotent stem cells from extraembryonic tissues of fetuses affected by monogenic diseases. Cell. Reprogram. 2015, 17, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Gao, W.Q. Concise Review: Patient-derived stem cell research for monogenic disorders. Stem Cells 2016, 34, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.W.; Croft, G.F.; Williams, D.J.; O’Keeffe, S.; Carrasco, M.A.; Davis, A.R.; Roybon, L.; Oakley, D.H.; Maniatis, T.; Henderson, C.E.; et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 2013, 33, 574–586. [Google Scholar] [CrossRef] [PubMed]

| Primers | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| 5S | TCGTCTGATCTCGGAAGCTAAGCA | AAAGCCTACAGCACCCGGTATT |
| OCT4 | AACCTGGAGTTTGTGCCAGGGTTT | TGAACTTCACCTTCCCTCCAACCA |
| SOX2 | AGAAGAGGAGAGAGAAAGAAAGGGAGAGA | GAGAGAGGCAAACTGGAATCAGGATCAAA |
| NANOG | CCTGAAGACGTGTGAAGATGAG | GCTGATTAGGCTCCAACCATAC |
| NCAM | ATGGAAACTCTATTAAAGTGAACCTG | TAGACCTCATACTCAGCATTCCAGT |
| Isl1 | GAATGGCATGCGGCATGTTTGA | CGCATTTGATCCCGTACAACCTGA |
| Lhx3 | TCGGACAAGGACAGCGTTCAG | TTTCCGCCAAGGAAGGCTCATCG |
| HB9 | CACCGAGACCCAGGTGAAGATTT | CCCTTCTGTTTCTCCGCTTCCT |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdocca, M.; Ciafrè, S.A.; Spitalieri, P.; Talarico, R.V.; Sanchez, M.; Novelli, G.; Sangiuolo, F. SMA Human iPSC-Derived Motor Neurons Show Perturbed Differentiation and Reduced miR-335-5p Expression. Int. J. Mol. Sci. 2016, 17, 1231. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081231
Murdocca M, Ciafrè SA, Spitalieri P, Talarico RV, Sanchez M, Novelli G, Sangiuolo F. SMA Human iPSC-Derived Motor Neurons Show Perturbed Differentiation and Reduced miR-335-5p Expression. International Journal of Molecular Sciences. 2016; 17(8):1231. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081231
Chicago/Turabian StyleMurdocca, Michela, Silvia Anna Ciafrè, Paola Spitalieri, Rosa Valentina Talarico, Massimo Sanchez, Giuseppe Novelli, and Federica Sangiuolo. 2016. "SMA Human iPSC-Derived Motor Neurons Show Perturbed Differentiation and Reduced miR-335-5p Expression" International Journal of Molecular Sciences 17, no. 8: 1231. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081231








