Suppression of Lipid Accumulation by Indole-3-Carbinol Is Associated with Increased Expression of the Aryl Hydrocarbon Receptor and CYP1B1 Proteins in Adipocytes and with Decreased Adipocyte-Stimulated Endothelial Tube Formation
Abstract
:1. Introduction
2. Results
2.1. Effects of I3C on Cell Viability and Lipid Accumulation in Mature Adipocytes
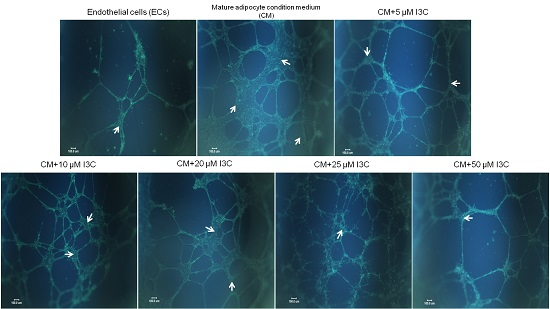
2.2. Effects of I3C on Adipocyte-Induced Tube Formation in ECs
2.3. Effects of I3C on Protein Expression by Mature Adipocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Biochemicals
4.2. Cell Culture
4.3. Cytotoxicity
4.4. Lipid Accumulation and Glycerol Release of 3T3-L1 Adipocyte
4.5. Tube Formation Assay
4.6. Assays of Nitric Oxide, VEGF, IL-6, and Matrix Metalloproteinase Activities
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rutkowski, J.M.; Davis, K.E.; Scherer, P.E. Mechanisms of obesity and related pathologies: The macro- and microcirculation of adipose tissue. FEBS J. 2009, 276, 5738–5746. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.L.; Bensley, J.G.; Wood-Bradley, R.J.; Cullen-McEwen, L.A.; Bertram, J.F.; Armitage, J.A. White adipocytes: More than just fat depots. Int. J. Biochem. Cell Biol. 2012, 44, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014, 40, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Adipose tissue vascularization: Its role in chronic inflammation. Curr. Diabetes Rep. 2011, 11, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J. Glucosinolates and their breakdown products in food and food plants. Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef] [PubMed]
- McDanell, R.; McLean, A.E.; Hanley, A.B.; Heaney, R.K.; Fenwick, G.R. Chemical and biological properties of indole glucosinolates (glucobrassicins): A review. Food Chem. Toxicol. 1988, 26, 59–70. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Wang, M.L.; Chan, M.H.; Chiu, Y.S.; Chen, Y.H. Antiobesity activities of indole-3-carbinol in high-fat-diet-induced obese mice. Nutrition 2011, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Park, S.; Lee, K.W.; Park, T. Indole-3-carbinol prevents diet-induced obesity through modulation of multiple genes related to adipogenesis, thermogenesis or inflammation in the visceral adipose tissue of mice. J. Nutr. Biochem. 2012, 23, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Um, S.J.; Park, T. Indole-3-carbinol directly targets SIRT1 to inhibit adipocyte differentiation. Int. J. Obes. 2013, 37, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.L.; Ganem, L.G.; Fernandez-Salguero, P.; Gonzalez, F.; Jefcoate, C.R. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J. Cell Sci. 1998, 111, 3311–3322. [Google Scholar] [PubMed]
- Shimba, S.; Todoroki, K.; Aoyagi, T.; Tezuka, M. Depletion of arylhydrocarbon receptor during adipose differentiation in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 1998, 249, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.C.; Carvajal-Gonzalez, J.M.; Rico-Leo, E.M.; Fernandez-Salguero, P.M. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J. Biol. Chem. 2009, 284, 25135–25148. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Zhang, L.; Murillo-Sauca, O.; Kim, J.; Kohrt, H.E.; Bui, J.D.; Sunwoo, J.B. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 12391–12396. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Lee, S.O.; Jin, U.H. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol. Sci. 2013, 135, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Wada, T.; Tezuka, M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentiation in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin. J. Cell Sci. 2001, 114, 2809–2817. [Google Scholar] [PubMed]
- Brodie, A.E.; Azarenko, V.A.; Hu, C.Y. 2,3,7,8-Tetrachlorodibenzo-ρ-dioxin (TCDD) inhibition of fat cell differentiation. Toxicol. Lett. 1996, 84, 55–59. [Google Scholar] [CrossRef]
- Phillips, M.; Enan, E.; Liu, P.C.; Matsumura, F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-ρ-dioxin. J. Cell Sci. 1995, 108, 395–402. [Google Scholar] [PubMed]
- Wang, M.L.; Lin, S.H.; Hou, Y.Y.; Chen, Y.H. Alpha-naphthoflavone increases lipid accumulation in mature adipocytes and enhances adipocyte-stimulated endothelial tube formation. Nutrients 2015, 7, 3166–3183. [Google Scholar] [CrossRef] [PubMed]
- Sul, H.S.; Smas, C.M.; Wang, D.; Chen, L. Regulation of fat synthesis and adipose differentiation. Prog. Nucleic Acid Res. Mol. Biol. 1998, 60, 317–345. [Google Scholar] [PubMed]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Schreiber, R.; Haemmerle, G.; Lass, A.; Fledelius, C.; Jacobsen, P.; Tornqvist, H.; Zechner, R.; Zimmermann, R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006, 281, 40236–40241. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Wakabayashi, N.; Misra, V.; Biswal, S.; Lee, G.H.; Agoston, E.S.; Yamamoto, M.; Kensler, T.W. NRF2 modulates aryl hydrocarbon receptor signaling: Influence on adipogenesis. Mol. Cell. Biol. 2007, 27, 7188–7197. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.V.; Su, G.H.; Reddy, J.K.; Simon, M.C.; Bradfield, C.A. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 1996, 93, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Tanos, R.; Murray, I.A.; Smith, P.B.; Patterson, A.; Perdew, G.H. Role of the Ah receptor in homeostatic control of fatty acid synthesis in the liver. Toxicol. Sci. 2012, 129, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Houlahan, K.E.; Prokopec, S.D.; Sun, R.X.; Moffat, I.D.; Lindén, J.; Lensu, S.; Okey, A.B.; Pohjanvirta, R.; Boutros, P.C. Transcriptional profiling of rat white adipose tissue response to 2,3,7,8-tetrachlorodibenzo-ρ-dioxin. Toxicol. Appl. Pharmacol. 2015, 288, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lindén, J.; Lensu, S.; Pohjanvirta, R. Effect of 2,3,7,8-tetrachlorodibenzo-ρ-dioxin (TCDD) on hormones of energy balance in a TCDD-sensitive and a TCDD-resistant rat strain. Int. J. Mol. Sci. 2014, 15, 13938–13966. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.S.; Chan, J.Y. Emerging role of Nrf2 in adipocytes and adipose biology. Adv. Nutr. 2013, 4, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xue, P.; Bai, Y.; Liu, D.; Woods, C.G.; Yarborough, K.; Fu, J.; Zhang, Q.; Sun, G.; Collins, S.; et al. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein beta during adipogenesis. Free Radic. Biol. Med. 2012, 52, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, V.; Lijnen, H.R. Angiogenesis and development of adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Richardson, R.L. Adipose tissue angiogenesis. J. Anim. Sci. 2004, 82, 925–934. [Google Scholar] [PubMed]
- Sarkanen, J.R.; Kaila, V.; Mannerstrom, B.; Raty, S.; Kuokkanen, H.; Miettinen, S.; Ylikomi, T. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng. Part A 2012, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kawakami, S.; Nakanishi, N. Fat depot-specific differences in angiogenic growth factor gene expression and its relation to adipocyte size in cattle. J. Vet. Med. Sci. 2010, 72, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Rega, G.; Kaun, C.; Demyanets, S.; Pfaffenberger, S.; Rychli, K.; Hohensinner, P.J.; Kastl, S.P.; Speidl, W.S.; Weiss, T.W.; Breuss, J.M.; et al. Vascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin m in human adipose tissue in vitro and in murine adipose tissue in vivo. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Shih, C.K.; Chang, H.P.; Chen, Y.H. Antiangiogenic activity of indole-3-carbinol in endothelial cells stimulated with activated macrophages. Food Chem. 2012, 134, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, T.A.; Broadbent, H.S. 1. The chemistry and pharmacology of indole-3-carbinol (indole-3-methanol) and 3-(methoxymethyl)indole. Curr. Med. Chem. 1998, 5, 337–352. [Google Scholar] [PubMed]
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.D.; Gescher, A.; Lamb, J.H.; Farmer, P.B.; Steward, W.P.; Williams, M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004, 10, 5233–5241. [Google Scholar] [CrossRef] [PubMed]
- Bjeldanes, L.F.; Kim, J.Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-ρ-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Riby, J.; Srivastava, P.; Bartholomew, J.; Denison, M.; Bjeldanes, L. Regulation of CYP1A1 by indolo[3,2-b]carbazole in murine hepatoma cells. J. Biol. Chem. 1995, 270, 22548–22555. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Tou, J.C.; Hong, C.; Kim, H.A.; Riby, J.E.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 2005, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, P.; Pugalendi, K.V.; Sankaran, M. Attenuation of hyperglycemia-mediated oxidative stress by indole-3-carbinol and its metabolite 3,3′-diindolylmethane in C57BL/6J mice. J. Physiol. Biochem. 2014, 70, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; She, W.; Wang, F.; Li, J.; Wang, J.; Jiang, W. 3,3′-Diindolylmethane alleviates steatosis and the progression of NASH partly through shifting the imbalance of Treg/Th17 cells to Treg dominance. Int. Immunopharmacol. 2014, 23, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, H.L.; Michnovicz, J.J.; Halper, M.; Miller, D.G.; Wong, G.Y.; Osborne, M.P. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol. Biomark. Prev. 1994, 3, 591–595. [Google Scholar]
- Rosen, C.A.; Bryson, P.C. Indole-3-carbinol for recurrent respiratory papillomatosis: Long-term results. J. Voice 2004, 18, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. PNAS 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-L.; Lin, S.-H.; Hou, Y.-Y.; Chen, Y.-H. Suppression of Lipid Accumulation by Indole-3-Carbinol Is Associated with Increased Expression of the Aryl Hydrocarbon Receptor and CYP1B1 Proteins in Adipocytes and with Decreased Adipocyte-Stimulated Endothelial Tube Formation. Int. J. Mol. Sci. 2016, 17, 1256. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081256
Wang M-L, Lin S-H, Hou Y-Y, Chen Y-H. Suppression of Lipid Accumulation by Indole-3-Carbinol Is Associated with Increased Expression of the Aryl Hydrocarbon Receptor and CYP1B1 Proteins in Adipocytes and with Decreased Adipocyte-Stimulated Endothelial Tube Formation. International Journal of Molecular Sciences. 2016; 17(8):1256. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081256
Chicago/Turabian StyleWang, Mei-Lin, Shyh-Hsiang Lin, Yuan-Yu Hou, and Yue-Hwa Chen. 2016. "Suppression of Lipid Accumulation by Indole-3-Carbinol Is Associated with Increased Expression of the Aryl Hydrocarbon Receptor and CYP1B1 Proteins in Adipocytes and with Decreased Adipocyte-Stimulated Endothelial Tube Formation" International Journal of Molecular Sciences 17, no. 8: 1256. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081256