Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis
Abstract
:1. Introduction
2. Serum and Glucocorticoid Regulated Kinase 1 (SGK1)-Dependent Regulation of Na+ Channels and Transporters
2.1. Epithelial Sodium Channel (ENaC)
2.2. Voltage-Gated Na+ Channel Nav1.5 (SCN5A)
2.3. Sodium Hydrogen Exchanger (NHE1 and NHE3)
2.4. Sodium-Chloride Symporter (NCC)
2.5. Na+-K+-2Cl− Cotransporter (NKCC2)
2.6. Sodium/Potassium-Adenosine Triphosphatase (Na+/K+-ATPase)
2.7. Type A Natriuretic Peptide Receptor (NPR-A)
3. Physiological Role of SGK1 in Na+ Transport
3.1. SGK1-Dependent Renal Na+ Reabsorption
3.2. SGK1-Dependent Renal Na+ Excretion
3.3. Aldosterone-Induced Salt Appetite
3.4. SGK1-Dependent Intestinal Sodium Absorption
3.5. SGK1-Dependent Lung Fluid Absorption
3.6. SGK1-Dependent Peripheral Na+ Transport
4. Pathological Role of SGK1 in Na+ Transport
4.1. Salt-Sensitive Hypertension
4.2. Edema with Diabetes Mellitus
4.3. Cardiac Dysfunction
4.4. Implantation Failure
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Webster, M.K.; Goya, L.; Firestone, G.L. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J. Biol. Chem. 1993, 268, 11482–11485. [Google Scholar] [PubMed]
- Webster, M.K.; Goya, L.; Ge, Y.; Maiyar, A.C.; Firestone, G.L. Characterization of SGK, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 1993, 13, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Waldegger, S.; Barth, P.; Raber, G.; Lang, F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc. Natl. Acad. Sci. USA 1997, 94, 4440–4445. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wesch, D.; Miranda, P.; Afonso-Oramas, D.; Althaus, M.; Castro-Hernandez, J.; Dominguez, J.; Morty, R.E.; Clauss, W.; Gonzalez-Hernandez, T.; de la Rosa, D.A.; et al. The neuronal-specific SGK1.1 kinase regulates delta-epithelial Na+ channel independently of PY motifs and couples it to phospholipase C signaling. Am. J. Physiol. Cell Physiol. 2010, 299, C779–C790. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mateos, E.; Brinkmeier, H.; Burks, T.N.; Mejias, R.; Files, D.C.; Steinberger, M.; Soleimani, A.; Marx, R.; Simmers, J.L.; Lin, B.; et al. Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol. Med. 2013, 5, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Keller-Wood, M.; Von Reitzenstein, M.; McCartney, J. Is the fetal lung a mineralocorticoid receptor target organ? Induction of cortisol-regulated genes in the ovine fetal lung, kidney and small intestine. Neonatology 2009, 95, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Notch, E.G.; Shaw, J.R.; Coutermarsh, B.A.; Dzioba, M.; Stanton, B.A. Morpholino gene knockdown in adult Fundulus heteroclitus: Role of SGK1 in seawater acclimation. PLoS ONE 2011, 6, e29462. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.R.; Bomberger, J.M.; VanderHeide, J.; LaCasse, T.; Stanton, S.; Coutermarsh, B.; Barnaby, R.; Stanton, B.A. Arsenic inhibits SGK1 activation of CFTR Cl− channels in the gill of killifish, Fundulus heteroclitus. Aquat. Toxicol. 2010, 98, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Roelants, F.M.; Baltz, A.G.; Trott, A.E.; Fereres, S.; Thorner, J. A protein kinase network regulates the function of aminophospholipid flippases. Proc. Natl. Acad. Sci. USA 2010, 107, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Schneiter, R. Ypk1, the yeast orthologue of the human serum- and glucocorticoid-induced kinase, is required for efficient uptake of fatty acids. J. Cell Sci. 2010, 123, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.R.; Buck, T.M.; Brodsky, J.L. Saccharomyces cerivisiae as a model system for kidney disease: What can yeast tell us about renal function? Am. J. Physiol. Ren. Physiol. 2011, 301, F1–F11. [Google Scholar] [CrossRef] [PubMed]
- Colabardini, A.C.; Brown, N.A.; Savoldi, M.; Goldman, M.H.S.; Goldman, G.H. Functional characterization of Aspergillus nidulans ypkA, a homologue of the mammalian kinase SGK. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Niles, B.J.; Powers, T. Plasma membrane proteins Slm1 and Slm2 mediate activation of the AGC kinase Ypk1 by TORC2 and sphingolipids in S. cerevisiae. Cell Cycle 2012, 11, 3745–3749. [Google Scholar] [CrossRef] [PubMed]
- Bakre, A.; Andersen, L.E.; Meliopoulos, V.; Coleman, K.; Yan, X.; Brooks, P.; Crabtree, J.; Tompkins, S.M.; Tripp, R.A. Identification of host kinase genes required for influenza virus replication and the regulatory role of microRNAs. PLoS ONE 2013, 8, e66796. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Huang, B.S.; Chen, A.; Ahmad, M.; White, R.A.; Leenen, F.H.H. Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience 2016, 314, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Limpens, J.H.; Spijker, S.; Vanderschuren, L.J.; Voorn, P. Stable immediate early gene expression patterns in medial prefrontal cortex and striatum after long-term cocaine self-administration. Addict. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-B.; Hsieh, M.-C.; Lai, C.-Y.; Cheng, J.-K.; Chau, Y.-P.; Ruan, T.; Chen, G.-D.; Peng, H.-Y. Modulation of nerve injury-induced HDAC4 cytoplasmic retention contributes to neuropathic pain in rats. Anesthesiology 2015, 123, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, T.Q.; Wang, Y.; Zhang, J.; Li, H.; Wang, K.J. Simulated bladder pressure stimulates human bladder smooth muscle cell proliferation via the PI3K/SGK1 signaling pathway. J. Urol. 2012, 188, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Sahin, P.; McCaig, C.; Jeevahan, J.; Murray, J.T.; Hainsworth, A.H. The cell survival kinase SGK1 and its targets FOXO3a and NDRG1 in aged human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fang, S.; Wan, C.; Kong, Q.; Wang, G.; Wang, S.; Zhang, H.; Zou, H.; Sun, B.; Sun, W.; et al. Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci. Rep. 2015, 5, 16548. [Google Scholar] [CrossRef] [PubMed]
- Rauz, S.; Walker, E.A.; Hughes, S.V.; Coca-Prados, M.; Hewison, M.; Murray, P.L.; Stewart, P.M. Serum- and glucocorticoid-regulated kinase isoform-1 and epithelial sodium channel subunits in human ocular ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1643–1651. [Google Scholar] [CrossRef]
- Rauz, S.; Walker, E.A.; Murray, P.I.; Stewart, P.M. Expression and distribution of the serum and glucocorticoid regulated kinase and the epithelial sodium channel subunits in the human cornea. Exp. Eye Res. 2003, 77, 101–108. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C.; He, Y.; Li, A.; Ni, W.; Sun, S.; Gu, X.; Li, J.; Li, H. miR-27a promotes apoptosis of cochlear sensory epithelium in Cx26 knockout mice. Front. Biosci. 2016, 21, 364–373. [Google Scholar]
- Kim, B.G.; Kim, J.Y.; Kim, M.; Kim, C.H.; Choi, J.Y.; Kim, S.H. Gene regulation by glucocorticoid in ENaC-mediated Na+ transport by middle ear epithelial cells. Laryngoscope 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lein, E.S.; Firestone, G.L. Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech. Dev. 2001, 103, 177–181. [Google Scholar] [CrossRef]
- Yu, X.B.; Lin, Q.; Qin, X.; Ruan, Z.; Zhou, J.H.; Lin, Z.F.; Su, Y.J.; Jian, Z. Serum and glucocorticoid kinase 1 promoted the growth and migration of non-small cell lung cancer cells. Gene 2016, 576, 339–346. [Google Scholar]
- Waldegger, S.; Klingel, K.; Barth, P.; Sauter, M.; Rfer, M.L.; Kandolf, R.; Lang, F. h-sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor β in human intestine. Gastroenterology 1999, 116, 1081–1088. [Google Scholar] [CrossRef]
- Kobayashi, T.; Deak, M.; Morrice, N.; Cohen, P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem. J. 1999, 344, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Klingel, K.; Warntges, S.; Bock, J.; Wagner, C.A.; Sauter, M.; Waldegger, S.; Kandolf, R.; Lang, F. Expression of cell volume-regulated kinase h-sgk in pancreatic tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G998–G1002. [Google Scholar] [PubMed]
- Yaylaoglu, M.B.; Agbemafle, B.M.; Oesterreicher, T.J.; Finegold, M.J.; Thaller, C.; Henning, S.J. Diverse patterns of cell-specific gene expression in response to glucocorticoid in the developing small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1041–G1050. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Speirs, H.J.L.; Seckl, J.R.; Brown, R.W. Sgk1 gene expression in kidney and its regulation by aldosterone: Spatio-temporal heterogeneity and quantitative analysis. J. Am. Soc. Nephrol. 2002, 13, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.T.; Campbell, D.G.; Morrice, N.; Auld, G.C.; Shpiro, N.; Marquez, R.; Peggie, M.; Bain, J.; Bloomberg, G.B.; Grahammer, F.; et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem. J. 2004, 384, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Pan, F.; Hao, Y.; Feng, W.; Song, H.; Zhu, D. SGK1 is regulated by metabolic-related factors in 3T3-L1 adipocytes and overexpressed in the adipose tissue of subjects with obesity and diabetes. Diabetes Res. Clin. Pract. 2013, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Panel, V.; Hayes, S.; Bagattin, A.; Meruvu, S.; Pandolfi, A.; Hugendubler, L.; Fejes-Toth, G.; Naray-Fejes-Toth, A.; Mueller, E. Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates adipocyte differentiation via forkhead Box O1. Mol. Endocrinol. 2010, 24, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Borst, O.; Schmidt, E.M.; Munzer, P.; Schonberger, T.; Towhid, S.T.; Elvers, M.; Leibrock, C.; Schmid, E.; Eylenstein, A.; Kuhl, D.; et al. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood 2012, 119, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Pelzl, L.; Fakhri, H.; Umbach, A.T.; Gawaz, M.; Paulmichl, M.; Lang, F. Sgk1 sensitive pendrin expression in murine platelets. Cell. Physiol. Biochem. 2013, 32, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.K.; Merches, K.; Bobbala, D.; Lang, F. AKT/SGK-sensitive phosphorylation of GSK3 in the regulation of L-selectin and perforin expression as well as activation induced cell death of T-lymphocytes. Biochem. Biophys. Res. Commun. 2012, 425, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Xuan, N.T.; Zahir, N.; Russo, A.; Yang, W.; Kuhl, D.; Faggio, C.; Shumilina, E.; Lang, F. Serum- and glucocorticoid-inducible kinase 1 sensitive NF-κB signaling in dendritic cells. Cell. Physiol. Biochem. 2014, 34, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Li, C.; Shen, Z.-X.; Zhang, W.-C.; Ai, T.-J.; Du, L.-J.; Zhang, Y.-Y.; Yao, G.-F.; Liu, Y.; Sun, S.; et al. Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-κB pathways. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Gu, S.C.; Yang, W.T.; Munzer, P.; Schaller, M.; Lang, F.; Stournaras, C.; Shumilina, E. Serum- and glucocorticoid-inducible kinase SGK1 regulates reorganization of actin cytoskeleton in mast cells upon degranulation. Am. J. Physiol. Cell Physiol. 2013, 304, C49–C55. [Google Scholar] [CrossRef] [PubMed]
- Alliston, T.N.; Gonzalez-Robayna, I.J.; Buse, P.; Firestone, G.L.; Richards, J.S. Expression and localization of serum/glucocorticoid-induced kinase in the rat ovary: Relation to follicular growth and differentiation. Endocrinology 2000, 141, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Aghajanian, H.K.; Lin, A.; Gerton, G.L. Signaling in sperm: Toward a molecular understanding of the acquisition of sperm motility in the mouse epididymis. Biol. Reprod. 2013, 89, 127. [Google Scholar] [CrossRef] [PubMed]
- Gerovska, D.; Arauzo-Bravo, M.J. Does mouse embryo primordial germ cell activation start before implantation as suggested by single-cell transcriptomics dynamics? Mol. Hum. Reprod. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, W.; Yan, W.L.; Peng, L.G. 116 Expression of insulin-like growth factor receptor (IGF2R), leptin receptor (LEPR) and serum- and glucocorticoid-regulated kinase 1 (SGK1) mRNA in human and bovine pregnancies. Reprod. Fertil. Dev. 2012, 25, 205. [Google Scholar] [CrossRef]
- Driver, P.M.; Rauz, S.; Walker, E.A.; Hewison, M.; Kilby, M.D.; Stewart, P.M. Characterization of human trophoblast as a mineralocorticoid target tissue. Mol. Hum. Reprod. 2003, 9, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Loffing, J.; Flores, S.Y.; Staub, O. Sgk kinases and their role in epithelial transport. Annu. Rev. Physiol. 2006, 68, 461–490. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Bohmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lehr, R.; Smallwood, A.M.; Ho, T.F.; Maley, K.; Randall, T.; Head, M.S.; Koretke, K.K.; Schnackenberg, C.G. Crystal structure of the kinase domain of serum and glucocorticoid-regulated kinase 1 in complex with AMP-PNP. Protein Sci. 2007, 16, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Voelkl, J. Therapeutic potential of serum and glucocorticoid inducible kinase inhibition. Expert Opin. Investig. Drugs 2013, 22, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Fairhall, E.A.; Charles, M.A.; Probert, P.M.; Wallace, K.; Gibb, J.; Ravindan, C.; Soloman, M.; Wright, M.C. Pancreatic B-13 Cell trans-differentiation to hepatocytes is dependent on epigenetic-regulated changes in gene expression. PLoS ONE 2016, 11, e0150959. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 2016, 6, 23084. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Schmidt, S.; Pouli, S.; Honisch, S.; Alkahtani, S.; Stournaras, C.; Lang, F. Membrane androgen receptor sensitive Na+/H+ exchanger activity in prostate cancer cells. FEBS Lett. 2014, 588, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, D.; Chen, S. SGK3 is an androgen-inducible kinase promoting prostate cancer cell proliferation through activation of p70 S6 kinase and up-regulation of cyclin D1. Mol. Endocrinol. 2014, 28, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Honisch, S.; Liu, G.X.; Schmidt, S.; Pantelakos, S.; Alkahtani, S.; Toulany, M.; Lang, F.; Stournaras, C. Inhibition of SGK1 enhances mAR-induced apoptosis in MCF-7 breast cancer cells. Cancer Biol. Ther. 2015, 16, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, V.K.; Attwood, K.; Campbell, M.J.; Smiraglia, D.J. Hormone stimulation of androgen receptor mediates dynamic changes in DNA methylation patterns at regulatory elements. Oncotarget 2015, 6, 42575–42589. [Google Scholar] [PubMed]
- Wehmeyer, L.; du Toit, A.; Lang, D.M.; Hapgood, J.P. Lipid raft- and protein kinase C-mediated synergism between glucocorticoid- and gonadotropin-releasing hormone signaling results in decreased cell proliferation. J. Biol. Chem. 2014, 289, 10235–10251. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, J.; Tsuruya, K.; Ikeda, H.; Noguchi, H.; Yotsueda, H.; Fujisaki, K.; Hirakawa, M.; Taniguchi, M.; Masutani, K.; Iida, M. Spironolactone inhibits hyperglycemia-induced podocyte injury by attenuating ROS production. Nephrol. Dial. Transplant. 2011, 26, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pollock, C.; Saad, S. Renal epidermal growth factor receptor: Its role in sodium and water homeostasis in diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2011, 38, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.; Gieryk, A.; Korostynski, M.; Piechota, M.; Wlazlo, E.; Przewlocki, R. Cell-type specific regulation of SGK1 isoforms by morphine and dexamethasone. Pharmacol. Rep. 2011, 63, 258. [Google Scholar]
- Chen, S.C.; Grigsby, C.L.; Law, C.S.; Ni, X.P.; Nekrep, N.; Olsen, K.; Humphreys, M.H.; Gardner, D.G. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J. Clin. Investig. 2009, 119, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Chraibi, A.; Renauld, S. PPARγ-induced stimulation of amiloride-sensitive sodium current in renal collecting duct principal cells is serum and insulin dependent. Cell. Physiol. Biochem. 2014, 33, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner-Reiter, M.H.; Kiefer, F.; Zeyda, M.; Stulnig, T.M.; Luger, A.; Vila, G. Strong association of serum- and glucocorticoid-regulated kinase 1 with peripheral and adipose tissue inflammation in obesity. Int. J. Obes. 2015, 39, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Della-Morte, D.; Coppola, A.; Capuani, B.; Lombardo, M.F.; Pacifici, F.; Ferrelli, F.; Arriga, R.; Mammi, C.; Federici, M.; et al. SGK-1 protects kidney cells against apoptosis induced by ceramide and TNF-α. Cell Death Dis. 2015, 6, e1890. [Google Scholar] [CrossRef] [PubMed]
- Quadri, S.; Siragy, H.M. (Pro)renin receptor contributes to regulation of renal epithelial sodium channel. J. Hypertens. 2016, 34, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A.; Babayeva, O.; Banerjee, S.; Zhong, W.; Francis, S.C. SGK1 is modulated by resistin in vascular smooth muscle cells and in the aorta following diet-induced obesity. Obesity 2016, 24, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Burgon, J.; Robertson, A.L.; Sadiku, P.; Wang, X.; Hooper-Greenhill, E.; Prince, L.R.; Walker, P.; Hoggett, E.E.; Ward, J.R.; Farrow, S.N.; et al. Serum and glucocorticoid-regulated kinase 1 regulates neutrophil clearance during inflammation resolution. J. Immunol. 2014, 192, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Umbach, A.T.; Chen, H.; Yan, J.; Fakhri, H.; Fajol, A.; Salker, M.S.; Spichtig, D.; Daryadel, A.; Wagner, C.A.; et al. Up-regulation of FGF23 release by aldosterone. Biochem. Biophys. Res. Commun. 2016, 470, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Gao, S.; Zhang, Y.; Wang, L.; Chen, X.; Wang, Y. miRNA and target gene expression in menstrual endometria and early pregnancy decidua. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zhu, B.; Sun, Y.; Xie, X.F. miR-155 modulates the progression of neuropathic pain through targeting SGK3. Int. J. Clin. Exp. Pathol. 2015, 8, 14374–14382. [Google Scholar] [PubMed]
- Kong, C.; Sun, L.; Zhang, M.; Ding, L.; Zhang, Q.; Cheng, X.; Diao, Z.; Yan, Q.; Zhang, H.; Fang, T.; et al. miR-133b reverses the hydrosalpinx-induced impairment of embryo attachment through down-regulation of SGK1. J. Clin. Endocrinol. Metab. 2016, 101, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Grahammer, F. Halting renal fibrosis: An unexpected role for mTORC2 signaling. Kidney Int. 2015, 88, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Salis, O.; Okuyucu, A.; Bedir, A.; Gor, U.; Kulcu, C.; Yenen, E.; Kilic, N. Antimetastatic effect of fluvastatin on breast and hepatocellular carcinoma cells in relation to SGK1 and NDRG1 genes. Tumour Biol. 2016, 37, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, W.; Wang, D.X. Insulin up-regulates epithelial sodium channel in LPS-induced acute lung injury model in rats by SGK1 activation. Injury 2012, 43, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, E.; Fornes, D.; Linenberg, I.; Powell, T.L.; Jansson, T.; Jawerbaum, A. A novel rat model of gestational diabetes induced by intrauterine programming is associated with alterations in placental signaling and fetal overgrowth. Mol. Cell. Endocrinol. 2016, 422, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.M.; Zhou, X.F.; Zhang, J.; Zheng, X.; Li, S.B.; Wei, Z.Q.; Liu, T.; Cheng, D.L.; Liu, P.; Song, K.; et al. DEPTOR suppresses the progression of esophageal squamous cell carcinoma and predicts poor prognosis. Oncotarget 2016, 7, 14188–14198. [Google Scholar] [PubMed]
- Fagerli, U.M.; Ullrich, K.; Stuhmer, T.; Holien, T.; Kochert, K.; Holt, R.U.; Bruland, O.; Chatterjee, M.; Nogai, H.; Lenz, G.; et al. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a prominent target gene of the transcriptional response to cytokines in multiple myeloma and supports the growth of myeloma cells. Oncogene 2011, 30, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Binger, K.J.; Linker, R.A.; Muller, D.N.; Kleinewietfeld, M. Sodium chloride, SGK1, and Th17 activation. Pflug. Arch. Eur. J. Physiol. 2015, 467, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Bhargava, A.; Mastroberardino, L.; Meijer, O.C.; Wang, J.; Buse, P.; Firestone, G.L.; Verrey, F.; Pearce, D. Epithelial sodium channel regulated by aldosterone-induced protein SGK. Proc. Natl. Acad. Sci. USA 1999, 96, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Fejes-Toth, G.; Frindt, G.; Naray-Fejes-Toth, A.; Palmer, L.G. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am. J. Physiol. Ren. Physiol. 2008, 294, F1298–F1305. [Google Scholar] [CrossRef] [PubMed]
- Bohmer, C.; Wagner, C.A.; Beck, S.; Moschen, I.; Melzig, J.; Werner, A.; Lin, J.T.; Lang, F.; Wehner, F. The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell. Physiol. Biochem. 2000, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; He, J.; Wang, D.X.; Deng, W.; Zhao, Y.; Ye, Y.; Feng, L.H. 17 β-Estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respir. Res. 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Amin, M.S.; El-Shahat, E.; Huang, B.S.; Tuana, B.S.; Leenen, F.H.H. Effects of central sodium on epithelial sodium channels in rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R222–R233. [Google Scholar] [CrossRef] [PubMed]
- Rauh, R.; Dinudom, A.; Fotia, A.B.; Paulides, M.; Kumar, S.; Korbmacher, C.; Cook, D.I. Stimulation of the epithelial sodium channel (ENaC) by the serum- and glucocorticoid-inducible kinase (Sgk) involves the PY motifs of the channel but is independent of sodium feedback inhibition. Pflug. Arch. Eur. J. Physiol. 2006, 452, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.E.; Kathpalia, P.P.; Chen, Y.; Thomas, S.V.; Noonan, E.J.; Pao, A.C. SGK1 regulation by miR-466g in cortical collecting duct cells. Am. J. Physiol. Ren. Physiol. 2016, 310, F1251–F1257. [Google Scholar] [CrossRef] [PubMed]
- Helms, M.N.; Fejes-Toth, G.; Naray-Fejes-Toth, A. Hormone-regulated transepithelial Na+ transport in mammalian CCD cells requires SGK1 expression. Am. J. Physiol. Ren. Physiol. 2003, 284, F480–F487. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Barbry, P.; Maiyar, A.C.; Rozansky, D.J.; Bhargava, A.; Leong, M.; Firestone, G.L.; Pearce, D. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am. J. Physiol. Ren. Physiol. 2001, 280, F303–F313. [Google Scholar]
- Arteaga, M.F.; Canessa, C.M. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am. J. Physiol. Ren. Physiol. 2005, 289, F90–F96. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.M.; Olson, D.R.; Thomas, B.C. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J. Biol. Chem. 2002, 277, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.B.; Ismail, N.A.S.; Caballero, A.G.; Land, S.C.; Wilson, S.M. Epithelial Na+ channel activity in human airway epithelial cells: The role of serum and glucocorticoid-inducible kinase 1. Br. J. Pharmacol. 2012, 166, 1272–1289. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Baines, D.L.; Wilson, S.M. The phosphorylation of endogenous Nedd4-2 In Na+-absorbing human airway epithelial cells. Eur. J. Pharmacol. 2014, 732, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntzsch, D.; Bergann, T.; Dames, P.; Fromm, A.; Fromm, M.; Davis, R.A.; Melzig, M.F.; Schulzke, J.D. The plant-derived glucocorticoid receptor agonist Endiandrin A acts as co-stimulator of colonic epithelial sodium channels (ENaC) via SGK-1 and MAPKs. PLoS ONE 2012, 7, e49426. [Google Scholar] [CrossRef] [PubMed]
- Debonneville, C.; Flores, S.Y.; Kamynina, E.; Plant, P.J.; Tauxe, C.; Thomas, M.A.; Munster, C.; Chraibi, A.; Pratt, J.H.; Horisberger, J.D.; et al. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 2001, 20, 7052–7059. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qi, D.; Wang, D.-X.; Deng, W.; Ye, Y.; Feng, L.-H.; Zhu, T.; Zhao, Y.; Zhang, C.-R. Insulin upregulates the expression of epithelial sodium channel in vitro and in a mouse model of acute lung injury: Role of mTORC2/SGK1 pathway. Exp. Cell Res. 2015, 331, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Vuagniaux, G.; Vallet, V.; Jaeger, N.F.; Hummler, E.; Rossier, B.C. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J. Gen. Physiol. 2002, 120, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.F.; Kabra, R.; Olson, D.R.; Piper, R.C.; Snyder, P.M. Hrs controls sorting of the epithelial Na+ channel between endosomal degradation and recycling pathways. J. Biol. Chem. 2010, 285, 30523–30530. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Shumilina, E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.M. Down-regulating destruction: Phosphorylation regulates the E3 ubiquitin ligase Nedd4-2. Sci. Signal. 2009, 2, pe41. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, K.; Yamamura, H.; Shimada, S.; Saito, T.; Hisanaga, S.; Taoka, M.; Isobe, T.; Ichimura, T. 14-3-3 Mediates phosphorylation-dependent inhibition of the interaction between the ubiquitin E3 ligase Nedd4-2 and epithelial Na+ channels. Biochemistry 2006, 45, 6733–6740. [Google Scholar] [PubMed]
- Staub, O.; Verrey, F. Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J. Am. Soc. Nephrol. 2005, 16, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.P.; Itani, O.A. New insights into epithelial sodium channel function in the kidney: Site of action, regulation by ubiquitin ligases, serum- and glucocorticoid-inducible kinase and proteolysis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kumar, S. Nedd4 and Nedd4-2: Closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010, 17, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kamynina, E.; Staub, O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na+ transport. Am. J. Physiol. Ren. Physiol. 2002, 283, F377–F387. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D. SGK1 regulation of epithelial sodium transport. Cell. Physiol. Biochem. 2003, 13, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Daidie, D.; Li, H.Y.; Pao, A.C.; LaGrange, L.P.; Wang, J.; Vandewalle, A.; Stockand, J.D.; Staub, O.; Pearce, D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol. Endocrinol. 2005, 19, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Melters, D.; Shih, I.C.; Wang, J.; Pearce, D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proc. Natl. Acad. Sci. USA 2009, 106, 7804–7809. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.V.; Kathpalia, P.P.; Rajagopal, M.; Charlton, C.; Zhang, J.N.; Eaton, D.C.; Helms, M.N.; Pao, A.C. Epithelial sodium channel regulation by cell surface-associated serum- and Glucocorticoid-regulated kinase 1. J. Biol. Chem. 2011, 286, 32074–32085. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Ziera, T.; Koo, E.; Ling, K.; Wang, J.; Borden, S.A.; Pearce, D. Scaffold protein connector enhancer of kinase suppressor of Ras isoform 3 (CNK3) coordinates assembly of a multiprotein epithelial sodium channel (ENaC)-regulatory complex. J. Biol. Chem. 2012, 287, 33014–33025. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Pearce, D.; Ziera, T. The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport. Mol. Cell. Endocrinol. 2012, 350, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Lu, M.; Pearce, D. Organization of the ENaC-regulatory machinery. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Rotin, D.; Staub, O. Role of the ubiquitin system in regulating ion transport. Pflug. Arch. Eur. J. Physiol. 2011, 461, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Edinger, R.S.; Lebowitz, J.; Li, H.; Alzamora, R.; Wang, H.; Johnson, J.P.; Hallows, K.R. Functional regulation of the epithelial Na+ channel by IκB kinase-β occurs via phosphorylation of the ubiquitin ligase Nedd4-2. J. Biol. Chem. 2009, 284, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, R.; Cheng, X.; Du, J. Antiapoptotic effect of serum and glucocorticoid-inducible protein kinase is mediated by novel mechanism activating IκB kinase. Cancer Res. 2005, 65, 457–464. [Google Scholar] [PubMed]
- Endo, T.; Kusakabe, M.; Sunadome, K.; Yamamoto, T.; Nishida, E. The kinase SGK1 in the endoderm and mesoderm promotes ectodermal survival by down-regulating components of the death-inducing signaling complex. Sci. Signal. 2011, 4, ra2. [Google Scholar] [CrossRef] [PubMed]
- Krueger, B.; Haerteis, S.; Yang, L.; Hartner, A.; Rauh, R.; Korbmacher, C.; Diakov, A. Cholesterol depletion of the plasma membrane prevents activation of the epithelial sodium channel (ENaC) by SGK1. Cell. Physiol. Biochem. 2009, 24, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Diakov, A.; Korbmacher, C. A novel pathway of epithelial sodium channel activation involves a serum- and glucocorticoid-inducible kinase consensus motif in the C terminus of the channel’s α-subunit. J. Biol. Chem. 2004, 279, 38134–38142. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.B.; Butterworth, M.B.; Peters, K.W.; Frizzell, R.A. AS160 modulates aldosterone-stimulated epithelial sodium channel forward trafficking. Mol. Biol. Cell 2010, 21, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.M.; Leng, Q.; Rinehart, J.; Wilson, F.H.; Kahle, K.T.; Hebert, S.C.; Lifton, R.P. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc. Natl. Acad. Sci. USA 2007, 104, 4025–4029. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.J.; Xu, B.E.; Deaton, S.L.; Cha, S.K.; Cheng, C.J.; Earnest, S.; Sengupta, S.; Juang, Y.C.; Stippec, S.; Xu, Y.D.; et al. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J. Biol. Chem. 2010, 285, 25161–25167. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Nelson, J.H.; McCormick, J.A.; Ellison, D.H. The WNK kinase network regulating sodium, potassium, and blood pressure. J. Am. Soc. Nephrol. 2011, 22, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Helms, M.N.; Yu, L.; Malik, B.; Kleinhenz, D.J.; Hart, C.M.; Eaton, D.C. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am. J. Physiol. Cell Physiol. 2005, 289, C717–C726. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, K.A.; Bugaj, V.; Mironova, E.; Stockand, J.D.; Pollock, J.S. NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct. Am. J. Physiol. Ren. Physiol. 2015, 308, F244–F251. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.; Toth, A.N.F. Gene regulation of ENaC subunits by serum- and glucocorticoid-inducible kinase-1. Am. J. Physiol. Ren. Physiol. 2005, 288, F505–F512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z.; Xia, X.F.; Reisenauer, M.R.; Rieg, T.; Lang, F.; Kuhl, D.; Vallon, V.; Kone, B.C. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel α. J. Clin. Investig. 2007, 117, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Yu, Z.Y.; Cruz, P.; Kong, Q.; Li, S.Y.; Kone, B.C. Epigenetics and the control of epithelial sodium channel expression in collecting duct. Kidney Int. 2009, 75, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhang, W. Regulation of αENaC transcription. Vitam. Horm. 2015, 98, 101–135. [Google Scholar] [PubMed]
- Wu, H.Y.; Chen, L.H.; Zhou, Q.L.; Zhang, W.Z. AF17 Facilitates dot1a nuclear export and upregulates ENaC-mediated Na+ transport in renal collecting duct cells. PLoS ONE 2011, 6, e27429. [Google Scholar] [CrossRef] [PubMed]
- Reisenauer, M.R.; Wang, S.W.; Xia, Y.; Zhang, W. Dot1a contains three nuclear localization signals and regulates the epithelial Na+ channel (ENaC) at multiple levels. Am. J. Physiol. Ren. Physiol. 2010, 299, F63–F76. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.D.; Zineh, I.; Burkley, B.; Gong, Y.; Langaee, T.Y.; Turner, S.T.; Chapman, A.B.; Boerwinkle, E.; Gums, J.G.; Cooper-Dehoff, R.M.; et al. Effects of genetic variation in H3K79 methylation regulatory genes on clinical blood pressure and blood pressure response to hydrochlorothiazide. J. Transl. Med. 2012, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Reisenauer, M.R.; Anderson, M.; Huang, L.; Zhang, Z.; Zhou, Q.; Kone, B.C.; Morris, A.P.; Lesage, G.D.; Dryer, S.E.; Zhang, W. AF17 competes with AF9 for binding to Dot1a to up-regulate transcription of epithelial Na+ channel α. J. Biol. Chem. 2009, 284, 35659–35669. [Google Scholar] [CrossRef] [PubMed]
- McTavish, N.; Getty, J.; Burchell, A.; Wilson, S.M. Glucocorticoids can activate the α-ENaC gene promoter independently of SGK1. Biochem. J. 2009, 423, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bezzerides, V.; Simonson, B.; Das, S.; Rosenzweig, A. Dual kinase control of cardiac sodium channel activity. Heart Rhythm. Conf. 2013, 10, S52–S53. [Google Scholar]
- Boehmer, C.; Wilhelm, V.; Palmada, M.; Wallisch, S.; Henke, G.; Brinkmeier, H.; Cohen, P.; Pieske, B.; Lang, F. Serum and glucocorticoid inducible kinases in the regulation of the cardiac sodium channel SCN5A. Cardiovasc. Res. 2003, 57, 1079–1084. [Google Scholar] [CrossRef]
- Das, S.; Aiba, T.; Rosenberg, M.; Hessler, K.; Xiao, C.Y.; Quintero, P.A.; Ottaviano, F.G.; Knight, A.C.; Graham, E.L.; Bostrom, P.; et al. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation 2012, 126, 2208–2219. [Google Scholar] [CrossRef] [PubMed]
- Marionneau, C.; Abriel, H. Regulation of the cardiac Na+ channel NaV1.5 by post-translational modifications. J. Mol. Cell. Cardiol. 2015, 82, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.A.; Saad, S.; Poronnik, P.; Fenton-Lee, C.A.; Polhill, T.S.; Pollock, C.A. The role of SGK-1 in angiotensin II-mediated sodium reabsorption in human proximal tubular cells. Nephrol. Dial. Transplant. 2008, 23, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Lang, F. New insights into role of serum- and glucorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure. Curr. Opin. Nephrol. Hypertens. 2005, 14, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Dynia, D.W.; Steinmetz, A.G.; Kocinsky, H.S. NHE3 function and phosphorylation are regulated by a calyculin A-sensitive phosphatase. Am. J. Physiol. Ren. Physiol. 2010, 298, F745–F753. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Sun, H.; Lang, F.; Yun, C.C. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am. J. Physiol. Cell Physiol. 2005, 289, C802–C810. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Lang, F.; Yun, C.C. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am. J. Physiol. Cell Physiol. 2007, 292, C396–C404. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.C. Concerted roles of SGK1 and the Na+/H+ exchanger regulatory factor 2 (NHERF2) in regulation of NHE3. Cell. Physiol. Biochem. 2003, 13, 29–40. [Google Scholar] [CrossRef] [PubMed]
- He, P.J.; Lee, S.J.; Lin, S.B.; Seidler, U.; Lang, F.; Fejes-Toth, G.; Naray-Fejes-Toth, A.; Yun, C.C. Serum- and glucocorticoid-induced kinase 3 in recycling endosomes mediates acute activation of Na+/H+ exchanger NHE3 by glucocorticoids. Mol. Biol. Cell 2011, 22, 3812–3825. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Pasham, V.; Ahmed, M.S.E.; Walker, B.; Szteyn, K.; Kuhl, D.; Metzler, B.; Alesutan, I.; Lang, F. Sgk1-dependent stimulation of cardiac Na+/H+ exchanger Nhe1 by dexamethasone. Cell. Physiol. Biochem. 2013, 32, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Lin, Y.; Alesutan, I.; Ahmed, M.S.; Pasham, V.; Mia, S.; Gu, S.; Feger, M.; Saxena, A.; Metzler, B.; et al. Sgk1 sensitivity of Na+/H+ exchanger activity and cardiac remodeling following pressure overload. Basic Res. Cardiol. 2012, 107, 236. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, L.; Li, J.; Huang, B.; Feng, J.; Li, C.; Wang, S.; The, E.; Liu, Y.; Yuan, T.; et al. Nucleoporin 35 regulates cardiomyocyte pH homeostasis by controlling Na+-H+ exchanger-1 expression. J. Mol. Cell Biol. 2015, 7, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.A.; Gadeau, A.P.; Fliegel, L.; Lopaschuk, G.; Mlih, M.; Abdulrahman, N.; Fillmore, N.; Mraiche, F. Na+/H+ exchanger isoform 1-induced osteopontin expression facilitates cardiomyocyte hypertrophy. PLoS ONE 2015, 10, e0123318. [Google Scholar] [CrossRef] [PubMed]
- Mlih, M.; Abdulrahman, N.; Gadeau, A.P.; Mohamed, I.A.; Jaballah, M.; Mraiche, F. Na+/H+ exchanger isoform 1 induced osteopontin expression in cardiomyocytes involves NFAT3/Gata4. Mol. Cell. Biochem. 2015, 404, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Schroth, J.; Lang, F.; Kuhl, D.; Uchida, S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am. J. Physiol. Ren. Physiol. 2009, 297, F704–F712. [Google Scholar] [CrossRef] [PubMed]
- Moes, A.D.; van der Lubbe, N.; Zietse, R.; Loffing, J.; Hoorn, E.J. The sodium chloride cotransporter SLC12A3: New roles in sodium, potassium, and blood pressure regulation. Pflug. Arch. Eur. J. Physiol. 2014, 466, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Al-Qusairi, L.; Donnelly, B.F.; Ronzaud, C.; Marciszyn, A.L.; Gong, F.; Chang, Y.P.C.; Butterworth, M.B.; Pastor-Soler, N.M.; Hallows, K.R.; et al. Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J. Clin. Investig. 2015, 125, 3433–3448. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H. Exploring the intricate regulatory network controlling the thiazide-sensitive NaCl cotransporter (NCC). Pflug. Arch. Eur. J. Physiol. 2011, 462, 767–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Lubbe, N.; Lim, C.H.; Fenton, R.A.; Meima, M.E.; Danser, A.H.J.; Zietse, R.; Hoorn, E.J. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011, 79, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhuang, J.; Gu, D.; Wang, H.; Cebotaru, L.; Guggino, W.B.; Cai, H. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J. Am. Soc. Nephrol. 2010, 21, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Angell, J.; Mitchell, R.; Ellison, D.H. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J. Clin. Investig. 2003, 111, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Rozansky, D.J.; Cornwall, T.; Subramanya, A.R.; Rogers, S.; Yang, Y.F.; David, L.L.; Zhu, X.M.; Yang, C.L.; Ellison, D.H. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J. Clin. Investig. 2009, 119, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.P.; Lagnaz, D.; Ronzaud, C.; Vazquez, N.; Ko, B.S.; Moddes, L.; Ruffieux-Daidie, D.; Hausel, P.; Koesters, R.; Yang, B.L.; et al. Nedd4-2 modulates renal Na+-Cl− cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J. Am. Soc. Nephrol. 2011, 22, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.; Mistry, A.C.; Hanson, L.; Mallick, R.; Wynne, B.M.; Thai, T.L.; Bailey, J.L.; Klein, J.D.; Hoover, R.S. Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am. J. Physiol. Ren. Physiol. 2013, 305, F645–F652. [Google Scholar] [CrossRef] [PubMed]
- Rotin, D.; Staub, O. Nedd4-2 and the regulation of epithelial sodium transport. Front. Physiol. 2012, 3, 212. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Ebrahim, A.; Siraskar, B.; Nasir, O.; Rexhepaj, R.; Amann, K.; Friedrich, B.; Risler, T.; Lang, F. Responses to diuretic treatment in gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1. Kidney Blood Press. Res. 2009, 32, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Klingel, K.; Wagner, C.A.; Stegen, C.; Warntges, S.; Friedrich, B.; Lanzendorfer, M.; Melzig, J.; Moschen, I.; Steuer, S.; et al. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2000, 97, 8157–8162. [Google Scholar] [CrossRef] [PubMed]
- Warntges, S.; Grone, H.J.; Capasso, G.; Lang, F. Cell volume regulatory mechanisms in progression of renal disease. J. Nephrol. 2001, 14, 319–326. [Google Scholar] [PubMed]
- Alvarez de la Rosa, D.; Gimenez, I.; Forbush, B.; Canessa, C.M. SGK1 activates Na+-K+-ATPase in amphibian renal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C492–C498. [Google Scholar] [CrossRef] [PubMed]
- Pao, A.C. SGK regulation of renal sodium transport. Curr. Opin. Nephrol. Hypertens. 2012, 21, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, I.; Henke, G.; Feng, Y.X.; Bohmer, C.; Vasilets, L.A.; Schwarz, W.; Lang, F. Stimulation of Xenopus oocyte Na+,K+ATPase by the serum and glucocorticoid-dependent kinase sgk1. Pflug. Arch.: Eur. J. Physiol. 2002, 444, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Summa, V.; Heitzmann, D.; Mordasini, D.; Vandewalle, A.; Feraille, E.; Zecevic, M. Short-term aldosterone action on Na,K-ATPase surface expression: Role of aldosterone-induced SGK1? Ann. N. Y. Acad. Sci. 2003, 986, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zecevic, M.; Heitzmann, D.; Camargo, S.M.R.; Verrey, F. SGK1 increases Na,K-ATP cell-surface expression and function in Xenopus laevis oocytes. Pflug. Arch. Eur. J. Physiol. 2004, 448, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Henke, G.; Setiawan, I.; Bohmer, C.; Lang, F. Activation of Na+/K+-ATPase by the serum and glucocorticoid-dependent kinase isoforms. Kidney Blood Press. Res. 2002, 25, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Waldegger, S.; Barth, P.; Forrest, J.N., Jr.; Greger, R.; Lang, F. Cloning of sgk serine-threonine protein kinase from shark rectal gland—A gene induced by hypertonicity and secretagogues. Pflug. Arch. Eur. J. Physiol. 1998, 436, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Waldegger, S.; Erdel, M.; Nagl, U.O.; Barth, P.; Raber, G.; Steuer, S.; Utermann, G.; Paulmichl, M.; Lang, F. Genomic organization and chromosomal localization of the human SGK protein kinase gene. Genomics 1998, 51, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; McCormick, J.A.; Prabaker, K.; Wang, J.; Pearce, D.; Gardner, D.G. Sgk1 mediates osmotic induction of NPR-A gene in rat inner medullary collecting duct cells. Hypertension 2004, 43, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Li, H.; Dong, W.X.; Krasinska, K.; Adams, C.; Alexandrova, L.; Chien, A.; Hallows, K.R.; Bhalla, V. Neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2) regulation by 14-3-3 protein binding at canonical serum and glucocorticoid kinase 1 (SGK1) phosphorylation sites. J. Biol. Chem. 2011, 286, 37830–37840. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Artunc, F.; Vallon, V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr. Opin. Nephrol. Hypertens. 2009, 18, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Pearce, D. Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol. Dial. Transplant. 2016, 31, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Robert-Nicoud, M.; Flahaut, M.; Elalouf, J.M.; Nicod, M.; Salinas, M.; Bens, M.; Doucet, A.; Wincker, P.; Artiguenave, F.; Horisberger, J.D.; et al. Transcriptome of a mouse kidney cortical collecting duct cell line: Effects of aldosterone and vasopressin. Proc. Natl. Acad. Sci. USA 2001, 98, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Fullerton, M.J.; Myles, K.; Purdy, T.M.; Funder, J.W.; Pearce, D.; Cole, T.J. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology 2001, 142, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Foo, R.; O’Shaughnessy, K.M.; Brown, M.J. Hyperaldosteronism: Recent concepts, diagnosis, and management. Postgrad. Med. J. 2001, 77, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, J.; Jones, K.T.; Ives, H.E.; Feldman, M.E.; Yao, L.J.; Shokat, K.M.; Ashrafi, K.; Pearce, D. mTOR complex-2 activates ENaC by phosphorylating SGK1. J. Am. Soc. Nephrol. 2010, 21, 811–818. [Google Scholar] [CrossRef] [PubMed]
- McDonald, F.J. A new SGK1 knockout mouse. Am. J. Physiol. Ren. Physiol. 2008, 294, F1296–F1297. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Padia, S.H. Intrarenal ghrelin receptors regulate ENaC-dependent sodium reabsorption by a cAMP-dependent pathway. Kidney Int. 2013, 84, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Samavat, S.; Ahmadpoor, P. Aldosterone, hypertension, and beyond. Iran. J. Kidney Dis. 2011, 5, 71–76. [Google Scholar] [PubMed]
- Fuster, D.G.; Bobulescu, I.A.; Zhang, J.; Wade, J.; Moe, O.W. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am. J. Physiol. Ren. Physiol. 2007, 292, F577–F585. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Wulff, P.; Huang, D.Y.; Loffing, J.; Volkl, H.; Kuhl, D.; Lang, F. Role of Sgk1 in salt and potassium homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R4–R10. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.A.; Parks, J.; Barati, M.T.; Lederer, E.D.; Clark, B.J.; Klein, J.D.; Khundmiri, S.J. Aldosterone regulates Na+, K+ ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.; Agapiou, D.J.; Chen, X.M.; Stevens, V.; Pollock, C.A. The role of Sgk-1 in the upregulation of transport proteins by PPARγ agonists in human proximal tubule cells. Nephrol. Dial. Transplant. 2009, 24, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.E.; Frindt, G.; Cheng, C.J.; Ng, M.; Kidwai, A.; Rashmi, P.; Lang, F.; Baum, M.; Palmer, L.G.; Pearce, D. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J. Clin. Investig. 2015, 125, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Faresse, N.; Lagnaz, D.; Debonneville, A.; Ismailji, A.; Maillard, M.; Fejes-Toth, G.; Naray-Fejes-Toth, A.; Staub, O. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am. J. Physiol. Ren. Physiol. 2012, 302, F977–F985. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Henke, G.; Embark, H.M.; Waldegger, W.; Palmada, M.; Bohmer, C.; Vallon, V. Regulation of channels by the serum and glucocorticoid-inducible kinase-implications for transport, excitability and cell proliferation. Cell. Physiol. Biochem. 2003, 13, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wulff, P.; Vallon, V.; Huang, D.Y.; Volkl, H.; Yu, F.; Richter, K.; Jansen, M.; Schlunz, M.; Klingel, K.; Loffing, J.; et al. Impaired renal Na+ retention in the sgk1-knockout mouse. J. Clin. Investig. 2002, 110, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Capasso, G.; Schwab, M.; Waldegger, S. Renal tubular transport and the genetic basis of hypertensive disease. Clin. Exp. Nephrol. 2005, 9, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zelenak, C.; Volkl, J.; Eichenmuller, M.; Regel, I.; Frohlich, H.; Kempe, D.; Jimenez, L.; Le Bellego, L.; Vergne, S.; et al. Hydration-sensitive gene expression in brain. Cell. Physiol. Biochem. 2011, 27, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.L.L.; Maiyar, A.C.; Kim, B.; O’Keeffe, B.A.; Firestone, G.L. Expression of the serum- and glueocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J. Biol. Chem. 2003, 278, 5871–5882. [Google Scholar] [CrossRef] [PubMed]
- Taruno, A.; Niisato, N.; Marunaka, Y. Intracellular calcium plays a role as the second messenger of hypotonic stress in gene regulation of SGK1 and ENaC in renal epithelial A6 cells. Am. J. Physiol. Ren. Physiol. 2008, 294, F177–F186. [Google Scholar] [CrossRef] [PubMed]
- Rozansky, D.J.; Wang, J.; Doan, N.; Purdy, T.; Faulk, T.; Bhargava, A.; Dawson, K.; Pearce, D. Hypotonic induction of SGK1 and Na+ transport in A6 cells. Am. J. Physiol. Ren. Physiol. 2002, 283, F105–F113. [Google Scholar] [CrossRef] [PubMed]
- Cat, A.N.D.; Ouvrard-Pascaud, A.; Tronche, F.; Clemessy, M.; Gonzalez-Nunez, D.; Farman, N.; Jaisser, F. Conditional transgenic mice for studying the role of the glucocorticoid receptor in the renal collecting duct. Endocrinology 2009, 150, 2202–2210. [Google Scholar]
- Lee, S.M.; Lee, Y.J.; Yoon, J.J.; Kang, D.G.; Lee, H.S. Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J. Ethnopharmacol. 2012, 141, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.M.; Leong, M.L.L.; Kim, B.; Wang, E.; Park, J.; Hemmings, B.A.; Firestone, G.L. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (SGK)by a p38 MAPK-dependent pathway. J. Biol. Chem. 2000, 275, 25262–25272. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Huang, D.Y.; Grahammer, F.; Wyatt, A.W.; Osswald, H.; Wulff, P.; Kuhl, D.; Lang, F. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R395–R401. [Google Scholar] [CrossRef] [PubMed]
- Farjah, M.; Roxas, B.P.; Geenen, D.L.; Danziger, R.S. Dietary salt regulates renal SGK1 abundance: Relevance to salt sensitivity in the Dahl rat. Hypertension 2003, 41, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Vallon, V. Mineralocorticoid-induced sodium appetite and renal salt retention: Evidence for common signaling and effector mechanisms. Nephron Physiol. 2014, 128, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Umbach, A.T.; Pathare, G.; Foller, M.; Brosens, J.J.; Artunc, F.; Lang, F. SGK1-dependent salt appetite in pregnant mice. Acta Physiol. 2011, 202, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Niisato, N.; Sawabe, Y.; Miyazaki, H.; Marunaka, Y. Aldosterone-induced abnormal regulation of ENaC and SGK1 in Dahl salt-sensitive rat. Biochem. Biophy. Res. Commun. 2006, 341, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Boini, K.M.; Osswald, H.; Friedrich, B.; Artunc, F.; Ullrich, S.; Rajamanickam, J.; Palmada, M.; Wulff, P.; Kuhl, D.; et al. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am. J. Physiol. Ren. Physiol. 2006, 291, F1264–F1273. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Boini, K.M.; Friedrich, B.; Metzger, M.; Just, L.; Osswald, H.; Wulff, P.; Kuhl, D.; Vallon, V.; Lang, F. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R935–R944. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Niisato, N.; Sawabe, Y.; Miyazaki, H.; Tokuda, S.; Nishio, K.; Yoshikawa, T.; Marunaka, Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biol. Int. 2007, 31, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirooka, Y.; Sunagawa, K. Miso (Japanese soybean paste) soup attenuates salt-induced sympathoexcitation and left ventricular dysfunction in mice with chronic pressure overload. Hukuoka Acta Med. 2014, 105, 48–56. [Google Scholar] [PubMed]
- Lu, X.; Li, M.; Zhou, L.; Jiang, H.; Wang, H.; Chen, J. Urinary serum- and glucocorticoid-inducible kinase SGK1 reflects renal injury in patients with immunoglobulin A nephropathy. Nephrology 2014, 19, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Coric, T.; Hernandez, N.; de la Rosa, D.A.; Shao, D.; Wang, T.; Canessa, C.M. Expression of ENaC and serum- and glucocorticoid-induced kinase 1 in the rat intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G663–G670. [Google Scholar] [CrossRef] [PubMed]
- Dames, P.; Bergann, T.; Fromm, A.; Bucker, R.; Barmeyer, C.; Krug, S.M.; Fromm, M.; Schulzke, J.-D. Interleukin-13 affects the epithelial sodium channel in the intestine by coordinated modulation of STAT6 and p38 MAPK activity. J. Physiol. 2015, 593, 5269–5282. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Romero, M.F. Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 2011, 73, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Musch, M.W.; Lucioni, A.; Chang, E.B. Aldosterone regulation of intestinal Na absorption involves SGK-mediated changes in NHE3 and Na+ pump activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G909–G919. [Google Scholar] [CrossRef] [PubMed]
- Keller-Wood, M.; Wood, C.E.; Hua, Y.; Zhang, D. Mineralocorticoid receptor expression in late-gestation ovine fetal lung. J. Soc. Gynecol. Investig. 2005, 12, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Koshy, S.; Folkesson, H.G. IL-1 β-induced cortisol stimulates lung fluid absorption in fetal guinea pigs via SGK-mediated Nedd4–2 inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L527–L533. [Google Scholar] [CrossRef] [PubMed]
- Wirbelauer, J.; Schmidt, B.; Klingel, K.; Cao, L.; Lang, F.; Speer, C.P. Serum and glucocorticoid-inducible kinase in pulmonary tissue of preterm fetuses exposed to chorioamnionitis. Neonatology 2008, 93, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Janer, C.; Pitkanen, O.M.; Suvari, L.; Turpeinen, U.; Palojarvi, A.; Andersson, S.; Helve, O. Duration of gestation and mode of delivery affect the genes of transepithelial sodium transport in pulmonary adaptation. Neonatology 2015, 107, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pondugula, S.R.; Raveendran, N.N.; Ergonul, Z.; Deng, Y.P.; Chen, J.; Sanneman, J.D.; Palmer, L.G.; Marcus, D.C. Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat. Physiol. Genom. 2006, 24, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.X.; Hu, G.H.; Liu, Z.H. Expression of ENaC, SGK1 and Nedd4 isoforms in the cochlea of guinea pig. Folia Histochem. Cytobiol. 2014, 52, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.D.; Sun, B.; Saxena, A.; Hopkins, P.N.; Jeunemaitre, X.; Brown, N.J.; Adler, G.K.; Williams, J.S. Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J. Hum. Hypertens. 2013, 27, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Galletti, F.; Barba, G. Altered renal handling of sodium in human hypertension: Short review of the evidence. Hypertension 2003, 41, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Boini, K.M.; Hennige, A.M.; Huang, D.Y.; Friedrich, B.; Palmada, M.; Boehmer, C.; Grahammer, F.; Artunc, F.; Ullrich, S.; Avram, D.; et al. Serum- and glucocorticoid-inducible kinase 1 mediates salt sensitivity of glucose tolerance. Diabetes 2006, 55, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.S.; Amin, M.S.; Leenen, F.H.H. The central role of the brain in salt-sensitive hypertension. Curr. Opin. Cardiol. 2006, 21, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Mansley, M.K.; Wilson, S.M. Dysregulation of epithelial Na+ absorption induced by inhibition of the kinases TORC1 and TORC2. Br. J. Pharmacol. 2010, 161, 1778–1792. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, Y.; Li, L.; Sun, Z.; Shen, Y.; Xing, J.; Li, M.; Su, D.; Liang, X. Hyperuricemia induces hypertension through activation of renal epithelial sodium channel (ENaC). Metabolism 2016, 65, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Auberson, M.; Hoffman-Pochon, N.; Vandewalle, A.; Kellenberger, S.; Schild, L. Epithelial Na+ channel mutants causing Liddle’s syndrome retain ability to respond to aldosterone and vasopressin. Am. J. Physiol. Ren. Physiol. 2003, 285, F459–F471. [Google Scholar] [CrossRef] [PubMed]
- Bertog, M.; Cuffe, J.E.; Pradervand, S.; Hummler, E.; Hartner, A.; Porst, M.; Hilgers, K.F.; Rossier, B.C.; Korbmacher, C. Aldosterone responsiveness of the epithelial sodium channel (ENaC) in colon is increased in a mouse model for Liddle’s syndrome. J. Physiol. 2008, 586, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Vitzthum, H.; Seniuk, A.; Schulte, L.H.; Muller, M.L.; Hetz, H.; Ehmke, H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J. Physiol. 2014, 592, 1139–1157. [Google Scholar] [CrossRef] [PubMed]
- Busjahn, A.; Luft, F.C. Twin studies in the analysis of minor physiological differences between individuals. Cell. Physiol. Biochem. 2003, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Busjahn, A.; Aydin, A.; Uhlmann, R.; Krasko, C.; Bahring, S.; Szelestei, T.; Feng, Y.X.; Dahm, S.; Sharma, A.M.; Luft, F.C.; et al. Serum- and glucocorticoid-regulated kinase (SGK1) gene and blood pressure. Hypertension 2002, 40, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Trochen, N.; Ganapathipillai, S.; Ferrari, P.; Frey, B.M.; Frey, F.J. Low prevalence of nonconservative mutations of serum and glucocorticoid-regulated kinase (SGK1) gene in hypertensive and renal patients. Nephrol. Dial. Transplant. 2004, 19, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- von Wowern, F.; Berglund, G.; Carlson, J.; Mansson, H.; Hedblad, B.; Melander, O. Genetic variance of SGK-1 is associated with blood pressure, blood pressure change over time and strength of the insulin-diastolic blood pressure relationship. Kidney Int. 2005, 68, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Busjahn, A.; Seebohm, G.; Maier, G.; Toliat, M.R.; Nurnberg, P.; Aydin, A.; Luft, F.C.; Lang, F. Association of the serum and glucocorticoid regulated kinase (sgk1) gene with QT interval. Cell. Physiol. Biochem. 2004, 14, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Lupescu, A.; Mota, M.; Mota, E.; Frey, A.; Simon, P.; Mertens, P.R.; Floege, J.; Luft, F.; Asante-Poku, S.; et al. Association of SGK1 gene polymorphisms with type 2 diabetes. Cell. Physiol. Biochem. 2008, 21, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wang, Y.; Wang, M.; Mu, J.J.; Liu, F.Q.; Wang, L.; Ren, K.Y.; Wang, D.; Yuan, Z.Y. Common variants in serum/glucocorticoid regulated kinase 1 (SGK1) and blood pressure responses to dietary sodium or potassium interventions: A family-based association STUDY. Kidney Blood Press. Res. 2015, 40, 424–434. [Google Scholar] [PubMed]
- Rexhepaj, R.; Boini, K.M.; Huang, D.Y.; Amann, K.; Artunc, F.; Wang, K.; Brosens, J.J.; Kuhl, D.; Lang, F. Role of maternal glucocorticoid inducible kinase SGK1 in fetal programming of blood pressure in response to prenatal diet. Am. J. Physiol. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R2008–R2013. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Huang, D.Y.; Vallon, V. SGK, renal function and hypertension. J. Nephrol. 2010, 23, S124–S129. [Google Scholar] [PubMed]
- Beltowski, J.; Rachanczyk, J.; Wlodarczyk, M. Thiazolidinedione-induced fluid retention: Recent insights into the molecular mechanisms. PPAR Res. 2013, 2013, 628628. [Google Scholar] [PubMed]
- Hong, G.Z.; Lockhart, A.; Davis, B.; Rahmoune, H.; Baker, S.; Ye, L.; Thompson, P.; Shou, Y.P.; O’Shaughnessy, K.; Ronco, P.; et al. PPARγ activation enhances cell surface ENaC α via up-regulation of SGK1 in human collecting duct cells. FASEB J. 2003, 17, 1966–1968. [Google Scholar] [PubMed]
- Panchapakesan, U.; Pollock, C.; Saad, S. Review article: Importance of the kidney proximal tubular cells in thiazolidinedione-mediated sodium and water uptake. Nephrology 2009, 14, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.; Stevens, V.A.; Wassef, L.; Poronnik, P.; Kelly, D.J.; Gilbert, R.E.; Pollock, C.A. High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney Int. 2005, 68, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, T.S.; Imig, J.D.; Staruschenko, A. Regulation of ENaC-mediated sodium reabsorption by peroxisome proliferator-activated receptors. PPAR Res. 2010, 2010, 703735. [Google Scholar] [CrossRef] [PubMed]
- Catela, C.; Kratsios, P.; Hede, M.; Lang, F.; Rosenthal, N. Serum and glucocorticoid-inducible kinase 1 (SGK1) is necessary for vascular remodeling during angiogenesis. Dev. Dyn. 2010, 239, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpashneh, E.; Poggioli, T.; Sarathchandra, P.; Lexow, J.; Monassier, L.; Terracciano, C.; Lang, F.; Damilano, F.; Zhou, J.Q.; Rosenzweig, A.; et al. Ablation of SGK1 impairs endothelial cell migration and tube formation leading to decreased neo-angiogenesis following myocardial infarction. PLoS ONE 2013, 8, e80268. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Aiba, T.; Hessler, K.; Rosenberg, M.; Xiao, C.Y.; Quintero, P.; Ottaviano, F.; Morissette, M.; delMonte, F.; Ellinor, P.; et al. Abstract 20389: Serum- and glucocorticoid-regulated kinase 1 contributes to adverse electrical remodeling by regulating cardiac Na+ channels. Circulation 2010, 122, A543. [Google Scholar]
- Feroze-Zaidi, F.; Fusi, L.; Takano, M.; Higham, J.; Salker, M.S.; Goto, T.; Edassery, S.; Klingel, K.; Boini, K.M.; Palmada, M.; et al. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 2007, 148, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Schneider, S.; Yang, W.; Liu, G.; Schmidt, E.M.; Schmid, E.; Mia, S.; Brucker, S.; Stournaras, C.; et al. TGFβ1 and SGK1-sensitive store-operated Ca2+ entry and Orai1 expression in endometrial Ishikawa cells. Mol. Hum. Reprod. 2013. [Google Scholar] [CrossRef]
- Fisher, S.J.; Giudice, L.C. SGK1: A fine balancing act for human pregnancy. Nat. Med. 2011, 17, 1348–1349. [Google Scholar] [CrossRef] [PubMed]
- Salker, M.S.; Christian, M.; Steel, J.H.; Nautiyal, J.; Lavery, S.; Trew, G.; Webster, Z.; Al-Sabbagh, M.; Puchchakayala, G.; Foller, M.; et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat. Med. 2011, 17, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Sun, X.F.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, H.Y.; Kong, S.B.; Wang, S.M.; Wang, H.M.; Wang, H.B.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Stournaras, C. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Horm.-Int. J. Endocrinol. Metab. 2013, 12, 160–171. [Google Scholar] [CrossRef]
- Artunc, F.; Amann, K.; Nasir, O.; Friedrich, B.; Sandulache, D.; Jahovic, N.; Risler, T.; Vallon, V.; Wulff, P.; Kuhl, D.; et al. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J. Mol. Med. 2006, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Ott, M.; Klingel, K.; Beck, S.; Melzig, J.; Friedrich, B.; Wild, K.N.; Broer, S.; Moschen, I.; Albers, A.; et al. Effects of the serine/threonine kinase SGK1 on the epithelial Na+ channel (ENaC) and CFTR: Implications for cystic fibrosis. Cell. Physiol. Biochem. 2001, 11, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Hoffmann, E.K. Role of ion transport in control of apoptotic cell death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar] [PubMed]
- Safa, K.; Ohori, S.; Borges, T.J.; Uehara, M.; Batal, I.; Shimizu, T.; Magee, C.N.; Belizaire, R.; Abdi, R.; Wu, C.; et al. Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory T cells. J. Am. Soc. Nephrol. 2015, 26, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yao, G.; Chen, W.; Tang, X.; Feng, X.; Sun, L. Exacerbation of lupus nephritis by high sodium chloride related to activation of SGK1 pathway. Int. Immunopharmacol. 2015, 29, 568–573. [Google Scholar] [CrossRef] [PubMed]


| Organ | Distribution of SGK1 | Reference |
|---|---|---|
| Brian | Hypothalamic nuclei | [16] |
| Ventral striatum | [17] | |
| Dorsal horn | [18] | |
| Dopamine neurons | [19] | |
| Cortical pyramidal neurones | [20] | |
| Blood-brain barrier (BBB) | [21] | |
| Eye | Ocular ciliary epithelium | [22] |
| Corneal endothelium | [23] | |
| Ear | Cochlear sensory epithelium | [24] |
| Apical membrane of middle ear epithelium | [25] | |
| Thymus | Epithelial cell | [2] |
| Heart | Heart chamber | [26] |
| Lung | Epithelial cell | [27] |
| Breast | Mammary gland | [28] |
| Liver | Epithelial cell | [29] |
| Pancreas | Pancreatic tissue | [30] |
| Intestine | Epithelium of the duodenum, jejunum, ileum, and colon | [31] |
| Kidney | Epithelium lining the nephrons (distal tubules, glomeruli, and inner medulla) | [32] |
| Bladder | Detrusor tissue | [19] |
| Muscle | Skeletal muscle | [33] |
| Adipose tissue | Adipocyte | [34,35] |
| Blood | Platelets | [36,37] |
| Immune system | T-lymphocytes | [38] |
| Dendritic cell | [39] | |
| Macrophage | [40] | |
| Mast cell | [41] | |
| Reproductive system | Several ovarian cell types including the oocytes of primordial follicles | [42] |
| Sperm | [43] | |
| Primordial germ cell | [44] | |
| Decidua | [45] | |
| Placental trophoblast | [46] |
| Na+ Channels and Transporters | Mediators | SGK1 Regulation | Possible Mechanism | Reference |
|---|---|---|---|---|
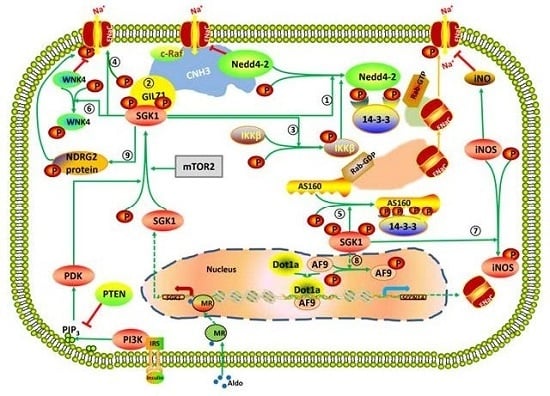
| ENaC | Nedd4-2/14-3-3 protein | SGK1 phosphorylates and sequesters the negative regulator Nedd4-2. Meanwhile, SGK1 recruits 14-3-3 to stabilize Nedd4-2 interacting with 14-3-3 | Nedd4-2 interacts with ENaC to induce ubiquitination and retrieval of ENaC channel; whereas 14-3-3 binds to Nedd4-2 and inhibits the interaction between Nedd4-2 and ENaC | [102,171] |
| iNOS | SGK1 phosphorylates iNOS | NO reduces the open probability of ENaC | [172] | |
| AF9-Dot1a complex | SGK1 phosphorylates AF9 and promotes Dot1a to dissociate from the α-ENaC promoter | AF9-Dot1a complex facilitates Dot1a to hypermethylate Lys79 of histone H3 and suppress α-ENaC transcription | [126] | |
| WNK4 | SGK1 phosphorylates WNK4 | WNK4 inhibits ENaC activity | [118] | |
| NDRG2 | SGK1 phosphorylates NDRG2 protein | NDRG2 stimulates ENaC activity and increase its surface expression | [33] | |
| Nav 1.5 | Nedd4-2 | SGK1 phosphorylates and inactivates Nedd4-2 | Nedd4-2 inhibits Nav1.5 activity | [135] |
| NHE1 | 14-3-3 protein | SGK1 recruits 14-3-3 binding | 14-3-3 facilitates SGK1 to phosphorylate and stimulate NHE1 | [143] |
| NHE3 | NHERF2 | SGK1 interacts with NHERF2 | NHERF2 tethers SGK1 and NHE3 to facilitate the phosphorylation of NHE3 | [141] |
| NCC | Nedd4-2 | SGK1 Phosphorylates Nedd4-2 and abrogates Nedd4-2-mediated inhibition | Nedd4-2 is co-located with NCC and involved in the ubiquitylation of NCC transporter | [156] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, Y.; Zhang, F.; Luo, Y.; Wang, L.; Huang, S.; Jin, F. Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis. Int. J. Mol. Sci. 2016, 17, 1307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081307
Lou Y, Zhang F, Luo Y, Wang L, Huang S, Jin F. Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis. International Journal of Molecular Sciences. 2016; 17(8):1307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081307
Chicago/Turabian StyleLou, Yiyun, Fan Zhang, Yuqin Luo, Liya Wang, Shisi Huang, and Fan Jin. 2016. "Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis" International Journal of Molecular Sciences 17, no. 8: 1307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081307