Chemokine-Like Receptor 1 mRNA Weakly Correlates with Non-Alcoholic Steatohepatitis Score in Male but Not Female Individuals
Abstract
:1. Introduction
2. Results
2.1. Hepatic Chemokine-Like Receptor 1 (CMKLR1) mRNA in the Human Liver
2.2. Hepatic CMKLR1 mRNA in Human Non-Alcoholic Fatty Liver Disease (NAFLD)
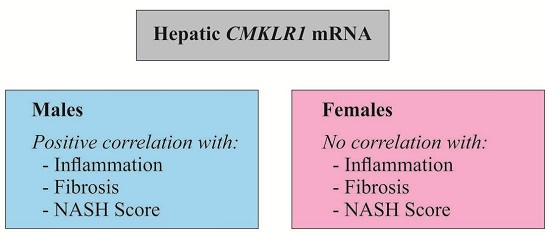
2.3. Hepatic CMKLR1 mRNA in Females
2.4. Hepatic CMKLR1 mRNA in Males
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Monitoring of Gene Expression by Real-Time RT-PCR
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buechler, C.; Wanninger, J.; Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 2011, 17, 2801–2811. [Google Scholar] [PubMed]
- Yeh, M.M.; Brunt, E.M. Pathological features of fatty liver disease. Gastroenterology 2014, 147, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Friis-Liby, I.; Aldenborg, F.; Jerlstad, P.; Rundstrom, K.; Bjornsson, E. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2004, 39, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Meder, W.; Wendland, M.; Busmann, A.; Kutzleb, C.; Spodsberg, N.; John, H.; Richter, R.; Schleuder, D.; Meyer, M.; Forssmann, W.G. Characterization of human circulating TIG2 as a ligand for the orphan receptor CHEMR23. FEBS Lett. 2003, 555, 495–499. [Google Scholar] [CrossRef]
- Yoshimura, T.; Oppenheim, J.J. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp. Cell Res. 2011, 317, 674–684. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Lu, Y.; Liu, S.; Zhang, Q.; Huang, J.; Zhu, R.; Yang, J.; Zhang, R.; Zhang, D.; et al. Identification of chemerin as a novel FXR target gene down-regulated in the progression of nonalcoholic steatohepatitis. Endocrinology 2013, 154, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Docke, S.; Lock, J.F.; Birkenfeld, A.L.; Hoppe, S.; Lieske, S.; Rieger, A.; Raschzok, N.; Sauer, I.M.; Florian, S.; Osterhoff, M.A.; et al. Elevated hepatic chemerin mrna expression in human non-alcoholic fatty liver disease. Eur. J. Endocrinol. 2013, 169, 547–557. [Google Scholar] [CrossRef]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C. Chemerin in liver diseases. Endocrinol. Metab. Syndr. 2014, 3, 1–6. [Google Scholar]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and CHEMR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Rouger, L.; Denis, G.R.; Luangsay, S.; Parmentier, M. CHEMR23 knockout mice display mild obesity but no deficit in adipocyte differentiation. J. Endocrinol. 2013, 219, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gruben, N.; Aparicio Vergara, M.; Kloosterhuis, N.J.; van der Molen, H.; Stoelwinder, S.; Youssef, S.; de Bruin, A.; Delsing, D.J.; Kuivenhoven, J.A.; van de Sluis, B.; et al. Chemokine-like receptor 1 deficiency does not affect the development of insulin resistance and nonalcoholic fatty liver disease in mice. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Haidl, I.D.; Zuniga, L.A.; Dranse, H.J.; Rourke, J.L.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012, 153, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, J.; Bauer, S.; Eisinger, K.; Weiss, T.S.; Walter, R.; Hellerbrand, C.; Schaffler, A.; Higuchi, A.; Walsh, K.; Buechler, C. Adiponectin upregulates hepatocyte CMKLR1 which is reduced in human fatty liver. Mol. Cell. Endocrinol. 2012, 349, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kukla, M.; Adamek, B.; Waluga, M.; Zalewska-Ziob, M.; Kasperczyk, J.; Gabriel, A.; Mazur, W.; Sobala-Szczygiel, B.; Buldak, R.J.; Zajecki, W.; et al. Hepatic chemerin and chemokine-like receptor 1 expression in patients with chronic hepatitis C. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Fallon, M.B. Gender and racial differences in nonalcoholic fatty liver disease. World J. Hepatol. 2014, 6, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Qin, S.; Wang, S.; Wang, X.; Zhang, Y.; Wang, J. Sex hormone affects the severity of non-alcoholic steatohepatitis through the MYD88-dependent Il-6 signaling pathway. Exp. Biol. Med. 2015, 240, 1279–1286. [Google Scholar] [CrossRef]
- Bauer, S.; Wanninger, J.; Schmidhofer, S.; Weigert, J.; Neumeier, M.; Dorn, C.; Hellerbrand, C.; Zimara, N.; Schaffler, A.; Aslanidis, C.; et al. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology 2011, 152, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Periz, A.; Horrillo, R.; Ferre, N.; Gronert, K.; Dong, B.; Moran-Salvador, E.; Titos, E.; Martinez-Clemente, M.; Lopez-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Guo, K.; Yi, L.; Gong, Y.; Huang, L.; Zhong, W. Resolvin e1 reduces hepatic fibrosis in mice with infection. Exp. Ther. Med. 2014, 7, 1481–1485. [Google Scholar] [PubMed]
- Pohl, R.; Rein-Fischboeck, L.; Meier, E.M.; Eisinger, K.; Krautbauer, S.; Buechler, C. Resolvin E1 and chemerin C15 peptide do not improve rodent non-alcoholic steatohepatitis. Exp. Mol. Pathol. 2015, 98, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Abdelmalek, M.F.; Suzuki, A.; Cummings, O.W.; Chojkier, M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014, 147, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rein-Fischboeck, L.; Krautbauer, S.; Eisinger, K.; Pohl, R.; Meier, E.M.; Weiss, T.S.; Buechler, C. Hepatic scavenger receptor BI is associated with type 2 diabetes but unrelated to human and murine non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2015, 467, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]




| Control | Borderline NASH | NASH | p-Values | |
|---|---|---|---|---|
| Males/Females | 16/17 | 22/25 | 24/14 | |
| Age | 58 (20–82) | 60 (24–84) | 66 (33–82) | 0.015 # |
| Body mass index (BMI) kg/m2 | 24.7 (18.3–30.5) | 28.0 (22.0–46.0) | 28.4 (21.0–57.7) | <0.001 *,# |
| Type 2 Diabetes | 0 | 4 | 11 | 0.01 # |
| Hypertension | 7 | 21 | 17 | |
| Hypercholesterolemia | 0 | 4 | 10 | |
| Alanine aminotransferase U/L | 21 (8–50) 32 | 35 (17–623) 36 | 32 (10–984) 35 | <0.001 *,# |
| Aspartate aminotransferase U/L | 23 (8–42) 27 | 31 (11–688) 35 | 30 (9–389) 34 | 0.014 * 0.012 # |
| Alkaline phosphatase U/L | 102 (46–203) 29 | 97 (37–444) 36 | 91 (45–826) 35 | |
| Bilirubin mg/dL | 0.6 (0.19–1.95) 30 | 0.56 (0.19–1.99) 37 | 0.53 (0.20–0.53) 36 | |
| Steatosis | 0 (0–0) | 2 (1–2) | 2.5 (1–3) | <0.001 *,#,& |
| Inflammation | 0 (0–0) | 0 (0–2) | 2 (0–3) | 0.005 * <0.001 #,& |
| Fibrosis | 0 (0–0) | 0 (0–2) | 2 (0–4) | 0.047 * <0.001 #,& |
| NASH Score | 0 (0–0) | 2 (1–4.5) | 6 (5–9) | <0.001 *,#,& |
| Scores | Description |
|---|---|
| Steatosis 0 | <5% steatosis |
| Steatosis 1 | 5%–33% steatosis |
| Steatosis 2 | >33%–66% steatosis |
| Steatosis 3 | >66% |
| Inflammation 0 | No foci/20 × field |
| Inflammation 1 | <2 foci/20 × field |
| Inflammation 2 | 2–4 foci/20 × field |
| Inflammation 3 | >4 foci/20 × field |
| Fibrosis 0 | No fibrosis |
| Fibrosis 1 | Zone 3 perisinusoidal/pericellular fibrosis; focally or extensively present |
| Fibrosis 2 | Zone 3 perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis |
| Fibrosis 3 | Zone 3 perisinusoidal/pericellular fibrosis and portal fibrosis with focal or extensive bridging fibrosis |
| Fibrosis 4 | Liver cirrhosis |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, M.; Meier, E.M.; Rein-Fischboeck, L.; Krautbauer, S.; Eisinger, K.; Aslanidis, C.; Pohl, R.; Weiss, T.S.; Buechler, C. Chemokine-Like Receptor 1 mRNA Weakly Correlates with Non-Alcoholic Steatohepatitis Score in Male but Not Female Individuals. Int. J. Mol. Sci. 2016, 17, 1335. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081335
Neumann M, Meier EM, Rein-Fischboeck L, Krautbauer S, Eisinger K, Aslanidis C, Pohl R, Weiss TS, Buechler C. Chemokine-Like Receptor 1 mRNA Weakly Correlates with Non-Alcoholic Steatohepatitis Score in Male but Not Female Individuals. International Journal of Molecular Sciences. 2016; 17(8):1335. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081335
Chicago/Turabian StyleNeumann, Maximilian, Elisabeth M. Meier, Lisa Rein-Fischboeck, Sabrina Krautbauer, Kristina Eisinger, Charalampos Aslanidis, Rebekka Pohl, Thomas S. Weiss, and Christa Buechler. 2016. "Chemokine-Like Receptor 1 mRNA Weakly Correlates with Non-Alcoholic Steatohepatitis Score in Male but Not Female Individuals" International Journal of Molecular Sciences 17, no. 8: 1335. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081335