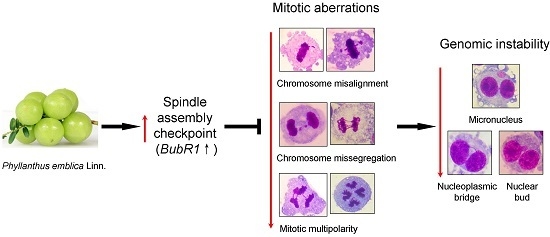
Phyllanthus emblica Fruit Extract Activates Spindle Assembly Checkpoint, Prevents Mitotic Aberrations and Genomic Instability in Human Colon Epithelial NCM460 Cells
Abstract
:1. Introduction
2. Results
2.1. Phyllanthus emblica Linn. (PE) Shows No Cytotoxicity
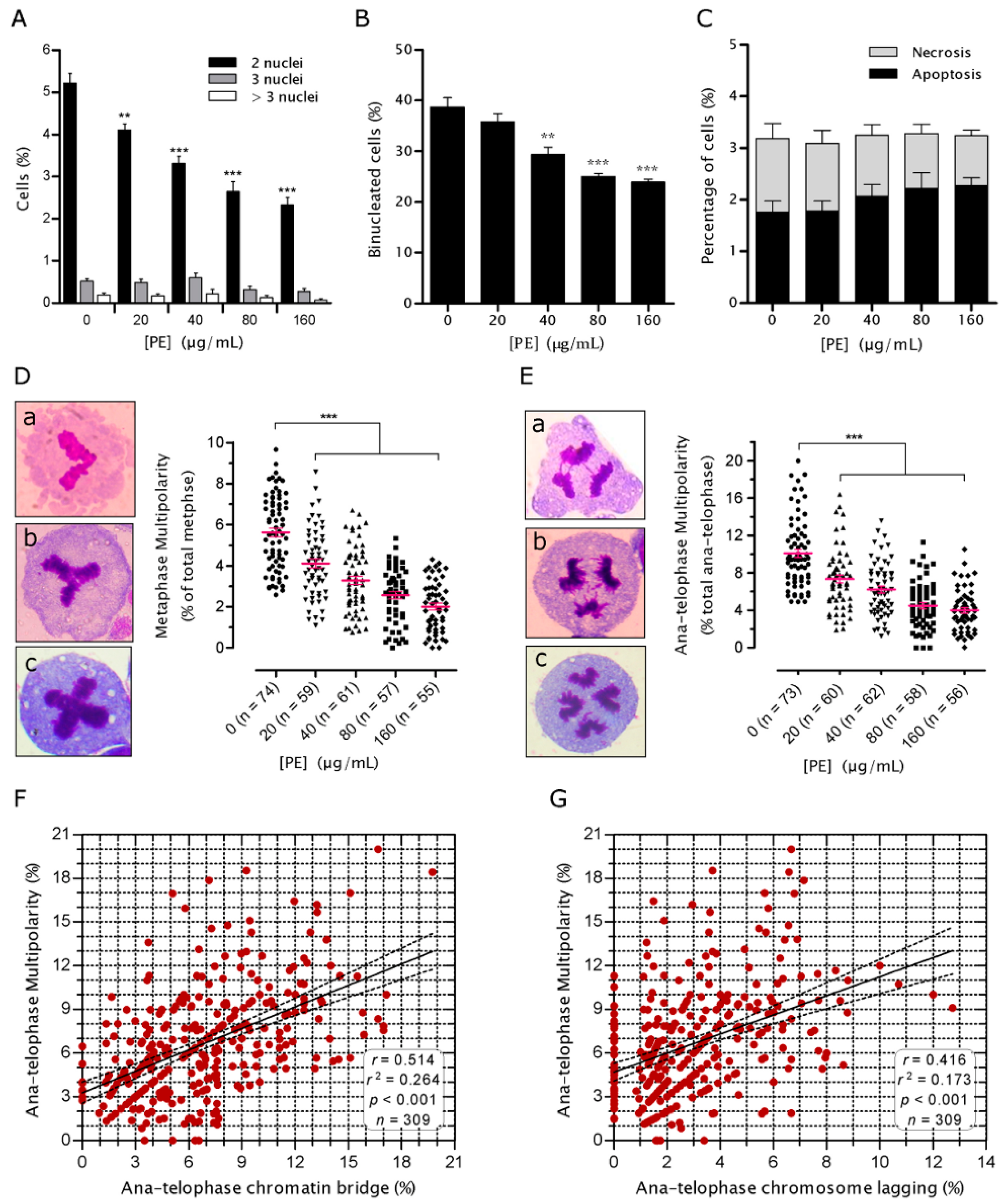
2.2. PE Decreases the Rate of Genomic Instability (GIN)
2.3. PE Prevents Defects in Chromosome Alignment and Segregation
2.4. PE Prevents Multinucleation and Multipolar Mitosis
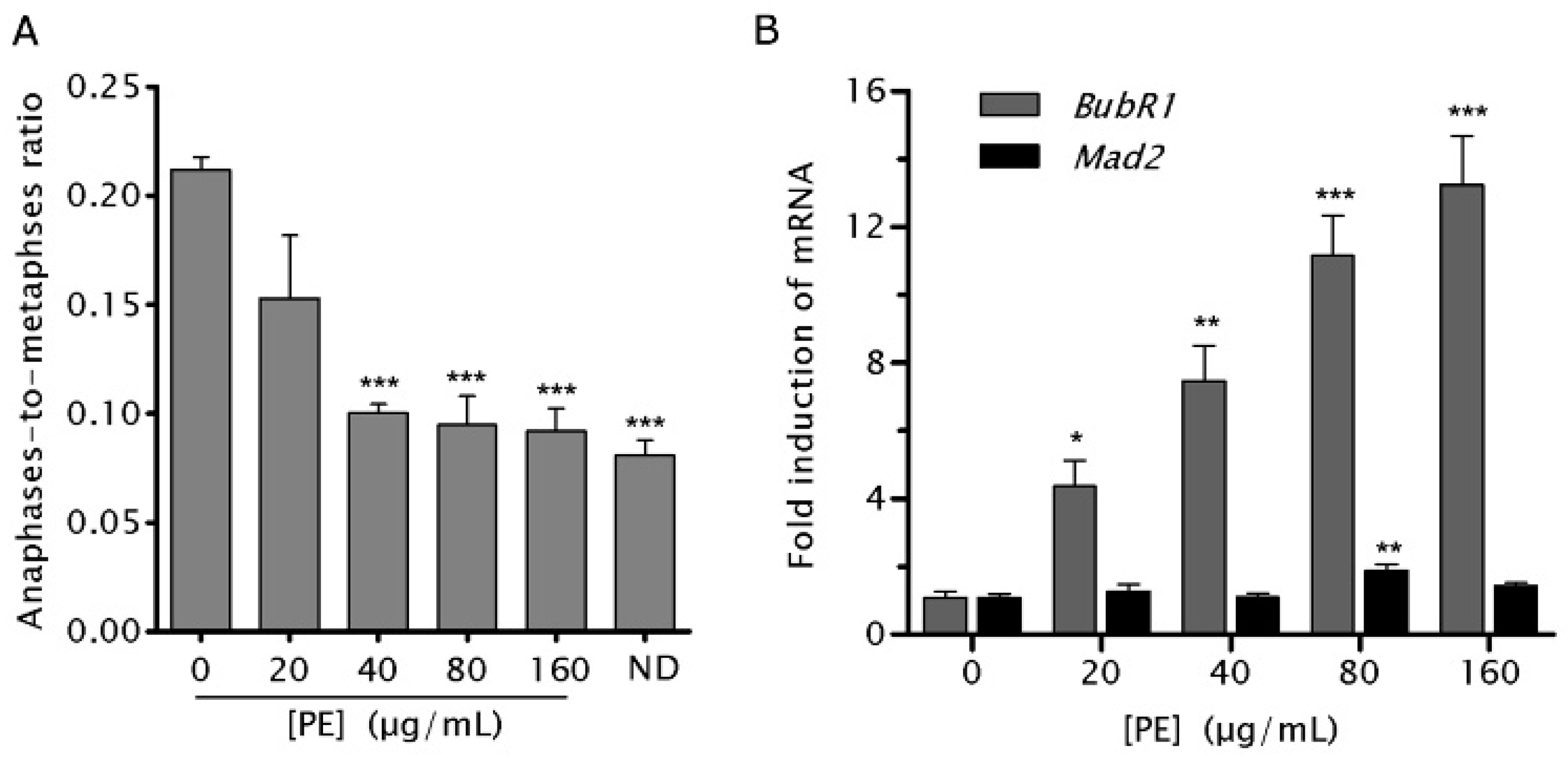
2.5. PE Activates the Spindle Assembly Checkpoint (SAC)
2.6. PE Enhances the Function of SAC
3. Discussion
4. Materials and Methods
4.1. PE Extraction
4.2. Cell Culture and Treatment
4.3. Cell Viability Analysis
4.4. Cytokinesis-Block Micronucleus (CBMN) Assay
4.5. Mitotic Index and Mitotic Aberration Analysis
4.6. Evaluation of Cell Death and Multinucleated Cells (MNC)
4.7. Determination of SAC Activity
4.8. Real-Time Quantitative Polymerase Chain Reaction (PCR)
4.9. Micronucleus (MN) Induced by Nocodazole (ND)
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMR | anaphase-to-metaphase ratio |
| BNC | binucleated cell |
| BubR1 | budding uninhibited by benzimidazoles related 1 |
| CB | chromatin bridge |
| CL | chromosome lagging |
| CMA | chromosome misalignment |
| GIN | genomic instability |
| Mad2 | mitotic arrest deficient 2 |
| MEF | mouse embryonic fibroblasts |
| MN | micronucleus |
| MNC | multinucleated cell |
| NB | nuclear bud |
| ND | nocodazole |
| NPB | nucleoplasmic bridge |
| PE | Phyllanthus emblica |
| SAC | spindle assembly checkpoint |
References
- Li, S. Bencao Gangmu; People’s Medical Publishing House: Beijing, China, 1977. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Burman, R.; Mansour, A.; Turki, Z.; Boulos, L.; Gullbo, J.; Göransson, U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013, 145, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Boldbaatar, D.; El-Seedi, H.R.; Findakly, M.; Jabri, S.; Javzan, B.; Choidash, B.; Göransson, U.; Hellman, B. Antigenotoxic and antioxidant effects of the Mongolian medicinal plant Leptopyrum fumarioides (L): An in vitro study. J. Ethnopharmacol. 2014, 155, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kristanc, L.; Kreft, S. European medicinal and edible plants associated with subacute and chronic toxicity part I: Plants with carcinogenic, teratogenic and endocrine-disrupting effects. Food Chem. Toxicol. 2016, 92, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Mirunalini, S.; Krishnaveni, M. Therapeutic potential of Phyllanthus emblica (amla): The ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 93–105. [Google Scholar] [CrossRef]
- Mathai, R.T.; Tonse, R.; Kalekhan, F.; Colin, M.D.; Prabhu, H.S.; Rao, S.; Baliga, M.S. Amla in the prevention of aging: Scientific validation of the ethnomedicinal claims. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Watson, R.R., Ed.; Academic Press: Waltham, MA, USA, 2015; pp. 29–35. [Google Scholar]
- Guo, X.; Ni, J.; Liu, X.; Xue, J.; Wang, X. Phyllanthus emblica L. fruit extract induces chromosomal instability and suppresses necrosis in human colon cancer cells. Int. J. Vitam. Nutr. Res. 2013, 83, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules 2016. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Noman, A.; Hossan, M.S.; Rashid, M.; Rahman, T.; Chowdhury, M.H.; Jahan, R. A survey of medicinal plants in two areas of Dinajpur district, Bangladesh including plants which can be used as functional foods. Am. Eurasian J. Sustain. Agric. 2009, 3, 862–876. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S.F.; Silkworth, W.T.; Nardi, I.K.; Nicholson, J.M.; Compton, D.A.; Cimini, D. The mitotic origin of chromosomal instability. Curr. Biol. 2014, 24, R148–R149. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Bakhoum, S.F.; Compton, D.A. Mechanisms of chromosomal instability. Curr. Biol. 2010, 20, R285–R295. [Google Scholar] [CrossRef] [PubMed]
- Lara-Gonzalez, P.; Westhorpe, F.G.; Taylor, S.S. The spindle assembly checkpoint. Curr. Biol. 2012, 22, R966–R980. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.W.; Taylor, M.H.; Hickey, R.D.; Newell, A.E.H.; Lenzi, M.L.; Olson, S.B.; Finegold, M.J.; Grompe, M. The ploidy-conveyor of mature hepatocytes as a source of genetic variation. Nature 2010, 467, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2006, 600, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Steigemann, P.; Wurzenberger, C.; Schmitz, M.H.; Held, M.; Guizetti, J.; Maar, S.; Gerlich, D.W. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 2009, 136, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Senovilla, L.; Jemaà, M.; Michaud, M.; Galluzzi, L.; Kepp, O.; Nanty, L.; Criollo, A.; Rello-Varona, S.; Manic, G. Multipolar mitosis of tetraploid cells: Inhibition by p53 and dependency on Mos. EMBO J. 2010, 29, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson, D. Classification of chromosome segregation errors in cancer. Chromosoma 2008, 117, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson, D.; Jonson, T.; Yu, C.; Martins, C.; Mandahl1, N.; Wiegant, J.; Jin, Y.; Mertens, F.; Jin, C. Centrosomal abnormalities, multipolar mitoses, and chromosomal instability in head and neck tumours with dysfunctional telomeres. Br. J. Cancer 2002, 87, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Stewénius, Y.; Gorunova, L.; Jonson, T.; Larsson, N.; Höglund, M.; Mandahl, N.; Mertens, F.; Mitelman, F.; Gisselsson, D. Structural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarity. Proc. Natl. Acad. Sci. USA 2005, 102, 5541–5546. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Toso, R.J.; Thrower, D.; Wilson, L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA 1993, 90, 9552–9556. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhang, Y.; Yi, Q.; Hou, H.; Xu, B.; Chu, L.; Huang, Y.; Zhang, W.; Fenech, M.; Shi, Q. Multiple origins of spontaneously arising micronuclei in HeLa cells: Direct evidence from long-term live cell imaging. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2008, 646, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.S.; Shuster, M.; Huang, X.; Gharaibeh, B.; Enyenihi, A.H.; Petersen, I.; Gollin, S.M. Chromosomal instability and cytoskeletal defects in oral cancer cells. Proc. Natl. Acad. Sci. USA 2000, 97, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Utani, K.-I.; Kohno, Y.; Okamoto, A.; Shimizu, N. Emergence of micronuclei and their effects on the fate of cells under replication stress. PLoS ONE 2010, 5, e10089. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; Li, Y.; Bosco, N.; Campbell, P.J.; Lange, T.d. Chromothripsis and kataegis induced by telomere crisis. Cell 2015, 163, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Ganem, N.J.; Godinho, S.A.; Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009, 460, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Silkworth, W.T.; Nardi, I.K.; Scholl, L.M.; Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 2009, 4, e6564. [Google Scholar] [CrossRef] [PubMed]
- Maiato, H.; Logarinho, E. Mitotic spindle multipolarity without centrsome amplification. Nat. Cell Biol. 2014, 16, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kapanidou, M.; Lee, S.; Bolanos-Garcia, V.M. BubR1 kinase: Rotection against aneuploidy and premature aging. Trends Mol. Med. 2015, 21, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, Q.; Liu, T.; Swamy, M.; Fang, Y.; Xie, S.; Mahmood, R.; Yang, Y.M.; Xu, M.; Rao, C.V. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004, 64, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Jeganathan, K.B.; Cameron, J.D.; Thompson, M.; Juneja, S.; Kopecka, A.; Kumar, R.; Jenkins, R.B.; Groen, P.C.D.; Roche, P.; et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004, 36, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, T.; Fang, Y.; Xie, S.; Huang, X.; Mahmood, R.; Ramaswamy, G.; Sakamoto, K.M.; Darzynkiewicz, Z.; Xu, M.; et al. BUBR1 deficiency results in abnormal megakaryopoiesis. Blood 2004, 103, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Lampson, M.A.; Kapoor, T.M. The human mitotic checkpoint protein BubR1 regulates chromosome–spindle attachments. Nat. Cell Biol. 2005, 7, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Matsumoto, Y.; Ikeuchi, T.; Saya, H.; Kajii, T.; Matsuura, S. BubR1 localizes to centrosomes and suppresses centrosome amplification via regulating Plk1 activity in interphase cells. Oncogene 2009, 28, 2806–2820. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Dawlaty, M.M.; Wijshake, T.; Jeganathan, K.B.; Malureanu, L.; Ree, J.H.V.; Ruben Crespo-Diaz, S.R.; Seaburg, L.; Shapiro, V.; Behfar, A.; et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat. Cell Biol. 2013, 15, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Poltanov, E.A.; Shikov, A.N.; Dorman, H.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical and antioxidant evaluation of Indian gooseberry (Emblica officinalis Gaertn., syn. Phyllanthus emblica L.) supplements. Phytother. Res. 2009, 23, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [PubMed]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, A.I.; Sologub, G.R.; Fomenko, L.A.; Zaichkina, S.I.; Kosyakova, N.I.; Bradbury, R.J. Effect of vitamin-antioxidant micronutrients on the frequency of spontaneous and in vitro γ-ray-induced micronuclei in lymphocytes of donors: The age factor. Carcinogenesis 1996, 17, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.; Jurányi, Z.; Székely, G.; Kocsis, Z.S.; Gundy, S. Relationship between spontaneous frequency of aneuploidy and cancer risk in 2145 healthy Hungarian subjects. Mutagenesis 2016. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.H.; York, T.P.; Juusola, J.; Ferreira-Gonzalez, A.; Maes, H.H.; Jackson-Cook, C. Genetic and environmental influences on spontaneous micronuclei frequencies in children and adults: A twin study. Mutagenesis 2011, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Nefic, H.; Handzic, I. The effect of age, sex, and lifestyle factors on micronucleus frequency in peripheral blood lymphocytes of the Bosnian population. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 753, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Yeager, M.; Zhou, W.; Wacholder, S. Detectable clonal mosaicism and its relationship to aging and cancer. Nat. Genet. 2012, 44, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Laurie, C.C.; Laurie, C.A.; Rice, K.; Doheny, K.F.; Zelnick, L.R.; McHugh, C.P.; Ling, H.; Hetrick, K.N.; Pugh, E.W.; Amos, C. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat. Genet. 2012, 44, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Machiela, M.J.; Zhou, W.; Sampson, J.N.; Dean, M.C.; Jacobs, K.B.; Black, A.; Brinton, L.A.; Chang, I.-S.; Chen, C.; Chen, C. Characterization of large structural genetic mosaicism in human autosomes. Am. J. Hum. Genet. 2015, 96, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Shendure, J.; Akey, J.M. The origins, determinants, and consequences of human mutations. Science 2015, 349, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Moyer, M.P.; Manzano, L.A.; Merriman, R.L.; Stauffer, J.S.; Tanzer, L.R. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell. Dev. Biol. Anim. 1996, 32, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Gruhne, B.; Sompallae, R.; Masucci, M. Three Epstein–Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene 2009, 28, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-H.; Xu, A.-M.; Chen, X.-F.; Li, D.-H.; Sun, M.-P.; Wang, Y.-J. Clinicopathologic significance of mitotic arrest defective protein 2 overexpression in hepatocellular carcinoma. Hum. Pathol. 2008, 39, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, H.; Ohgaki, H.; Merlo, A.; Shen, Z. Alterations of BCCIP, a BRCA2 interacting protein, in astrocytomas. BMC Cancer 2009. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.D.; Brady, A.L.; Stack, H.F.; Brockman, H.E. Antimutagenicity profiles for some model compounds. Mutat. Res. Rev. Genet. Toxicol. 1990, 238, 57–85. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, X. Phyllanthus emblica Fruit Extract Activates Spindle Assembly Checkpoint, Prevents Mitotic Aberrations and Genomic Instability in Human Colon Epithelial NCM460 Cells. Int. J. Mol. Sci. 2016, 17, 1437. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091437
Guo X, Wang X. Phyllanthus emblica Fruit Extract Activates Spindle Assembly Checkpoint, Prevents Mitotic Aberrations and Genomic Instability in Human Colon Epithelial NCM460 Cells. International Journal of Molecular Sciences. 2016; 17(9):1437. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091437
Chicago/Turabian StyleGuo, Xihan, and Xu Wang. 2016. "Phyllanthus emblica Fruit Extract Activates Spindle Assembly Checkpoint, Prevents Mitotic Aberrations and Genomic Instability in Human Colon Epithelial NCM460 Cells" International Journal of Molecular Sciences 17, no. 9: 1437. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091437