The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging
Abstract
:1. Introduction
2. Results and Discussion
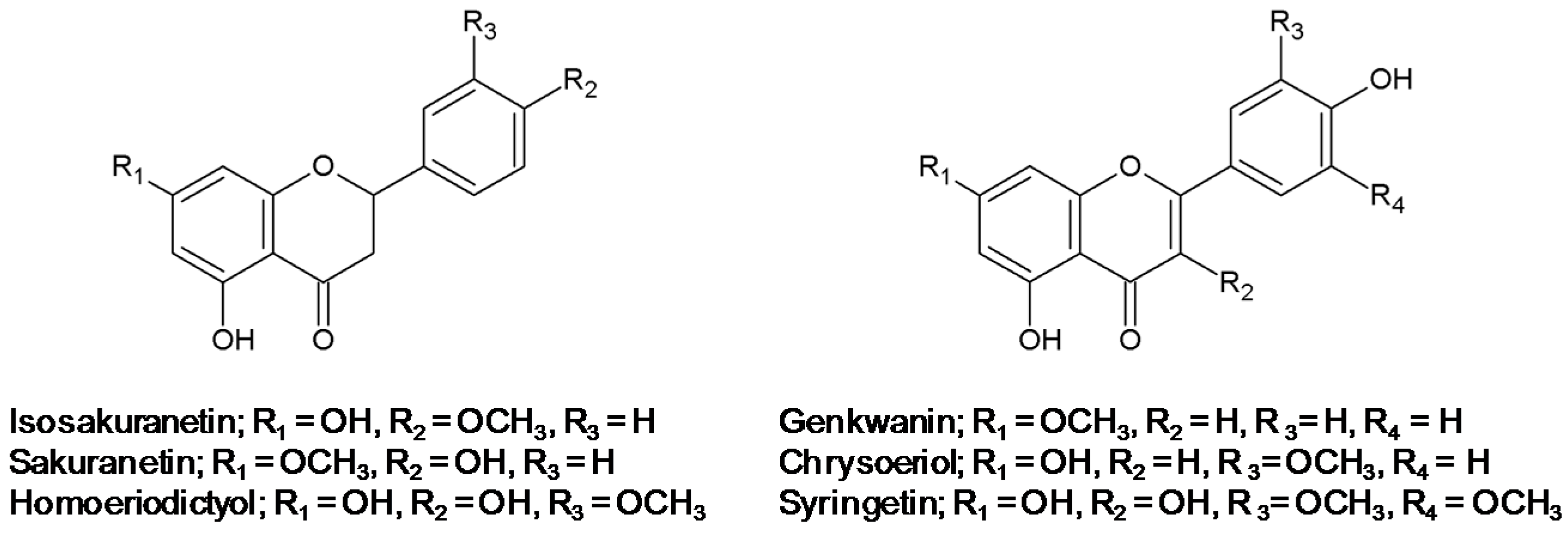
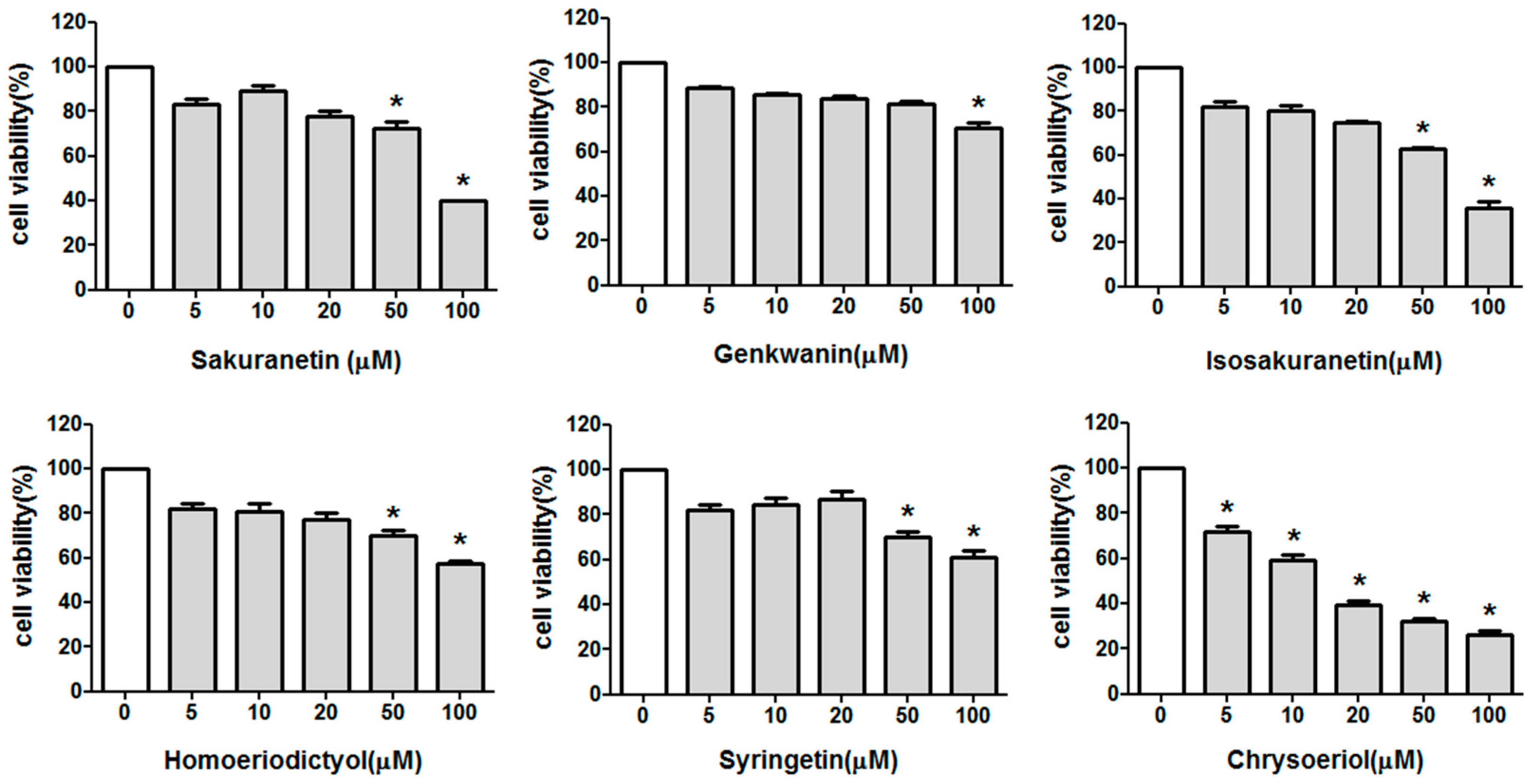
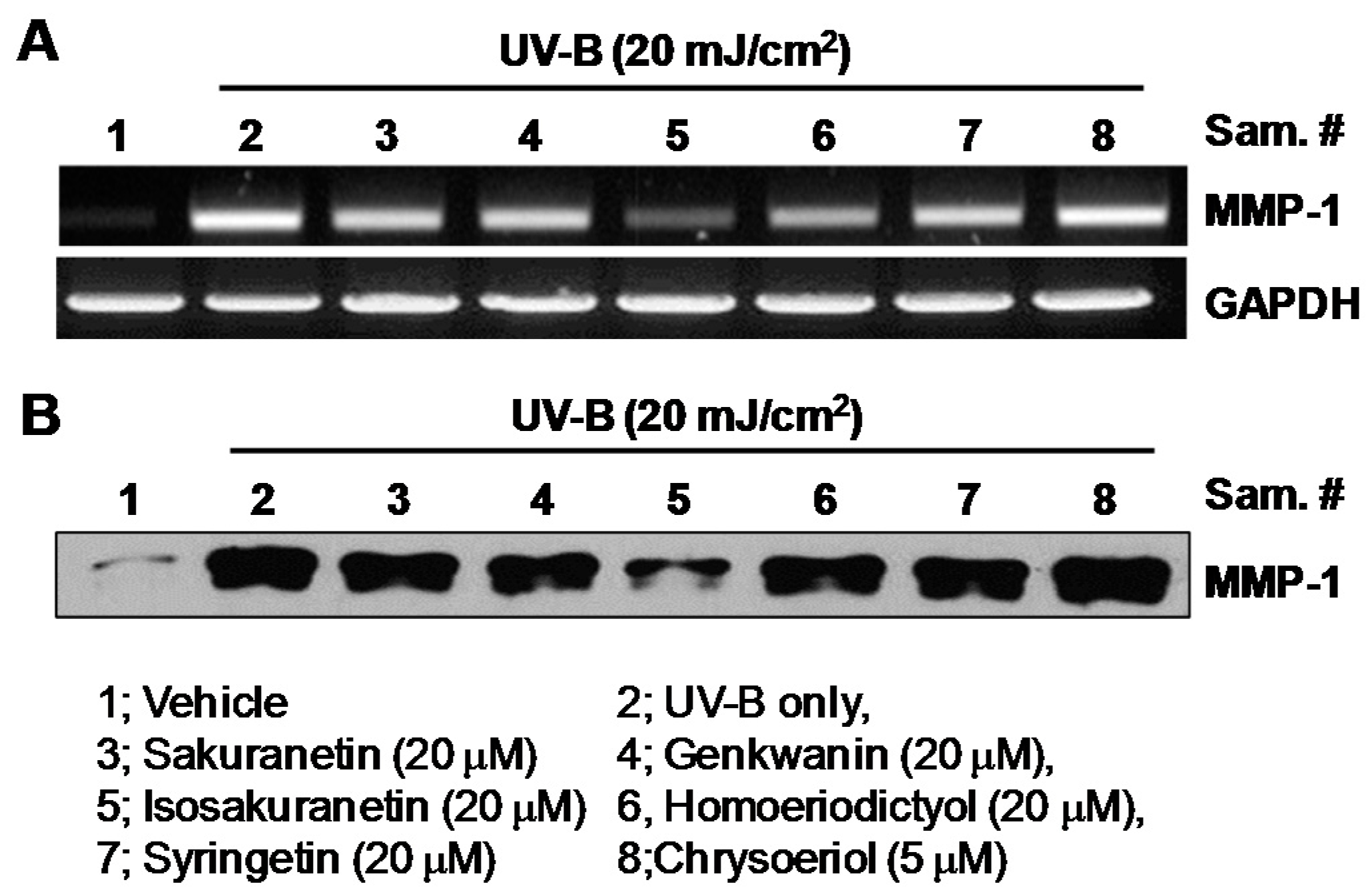
2.1. Effect of Methoxyflavonoids on Ultraviolet (UV)-B-Induced Matrix Metalloproteinase-1 (MMP-1) Expression in Human Keratinocytes
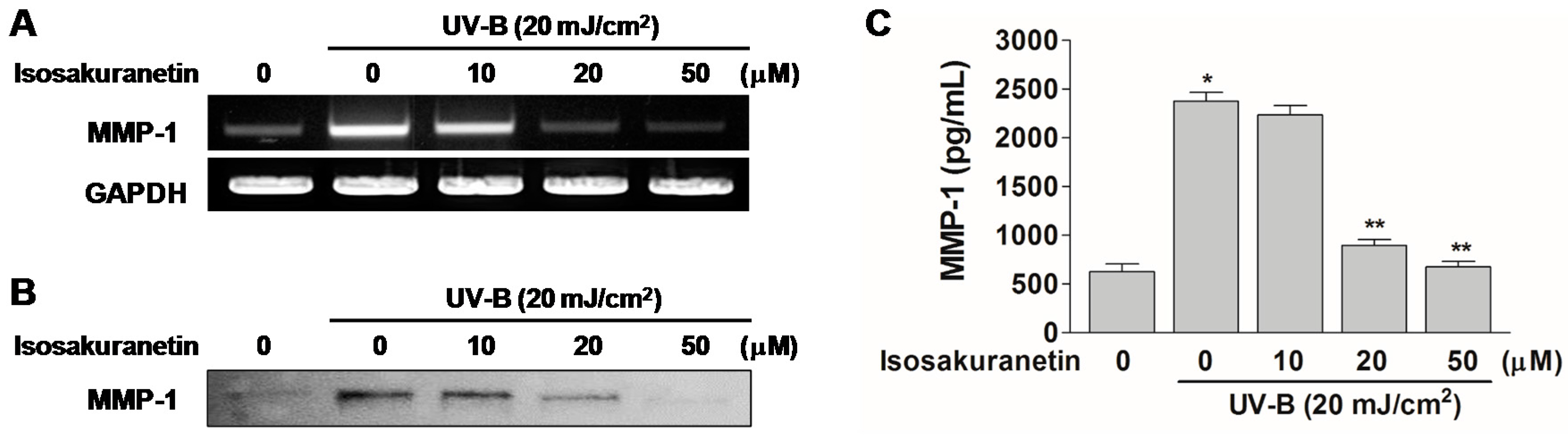
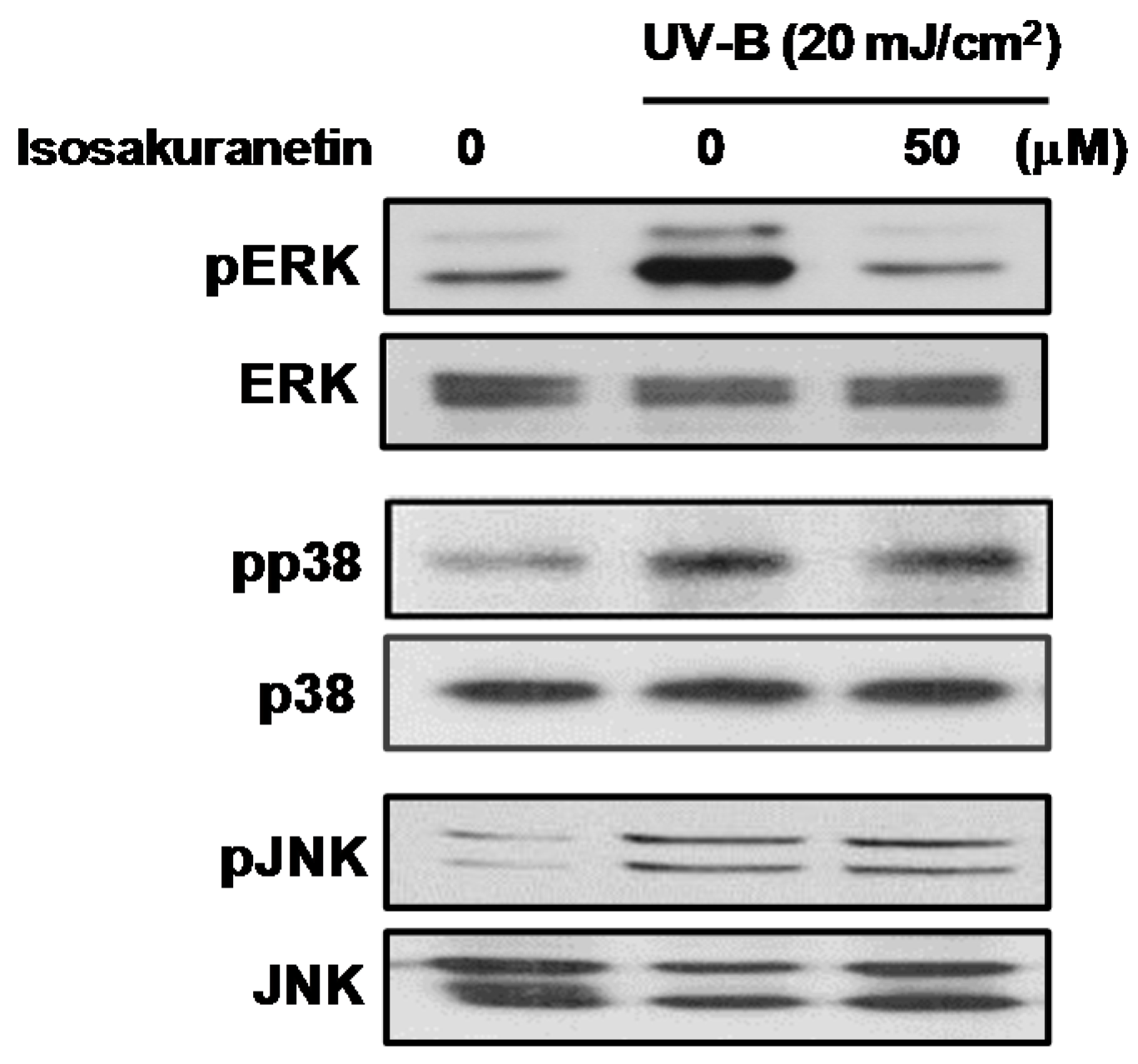
2.2. Isosakuranetin Inhibits UV-B-Induced MMP-1 Expression through the Suppression of ERK1/2 Kinase Pathways
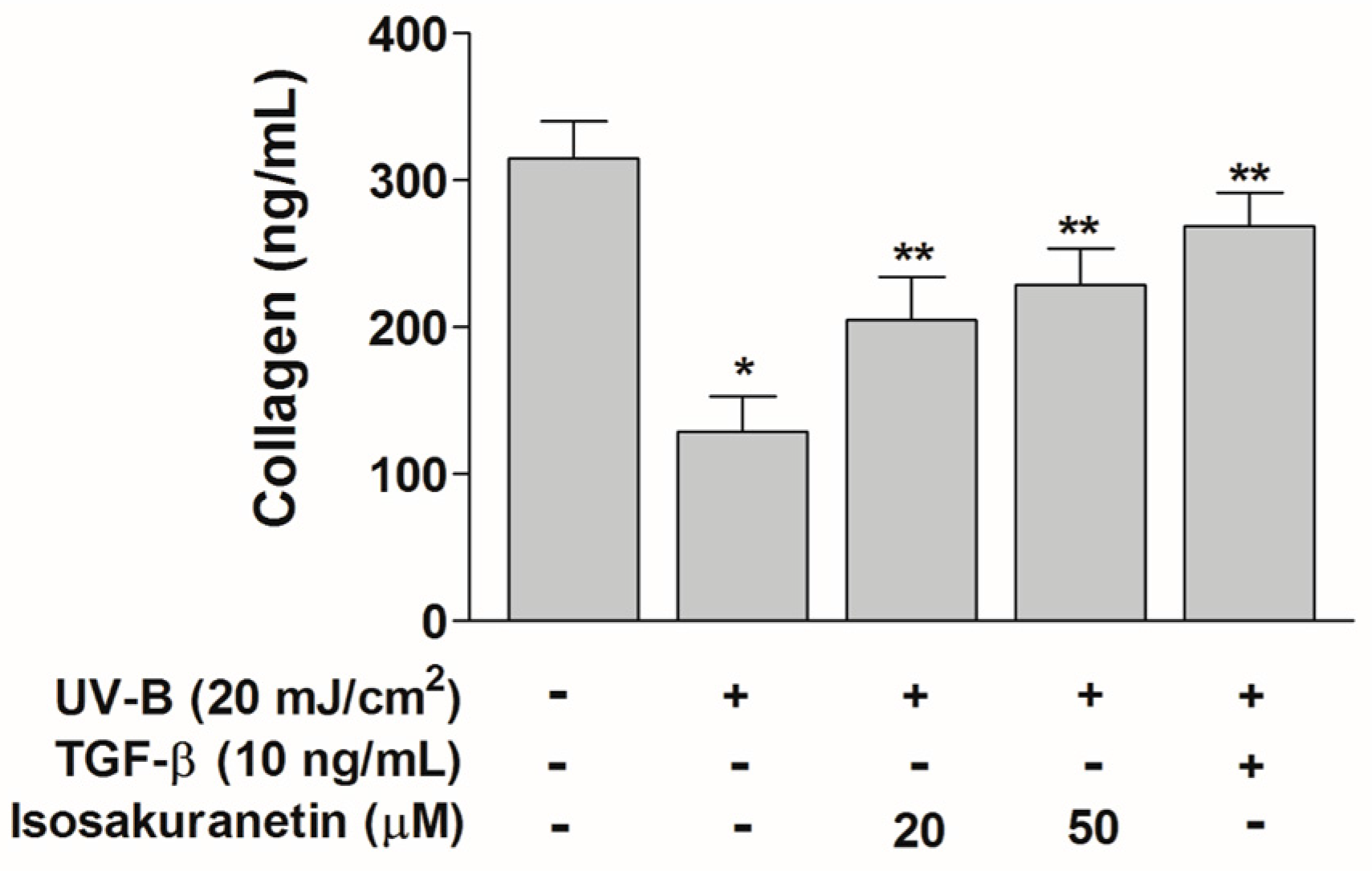
2.3. Isosakuranetin Protects Collagen Degradation from UV-B Radiation
3. Materials and Methods
3.1. Cell Culture and Reagents
3.2. Cell Viability Assay
3.3. UV-B Irradiation
3.4. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
3.5. Western Blot Analysis
3.6. Enzyme-Linked Immunosorbent Assay
3.7. Measurement of Procollagen
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Nam, K.W.; Hyun, J.W.; Chae, S. Phloroglucinol reduces photodamage in hairless mice via matrix metalloproteinase activity through MAPK pathway. Photochem. Photobiol. 2016, 92, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chiang, A.N.; Liu, H.N.; Chang, Y.T. EGb-761 prevents ultraviolet B-induced photoaging via inactivation of mitogen-activated protein kinases and proinflammatory cytokine expression. J. Dermatol. Sci. 2014, 75, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Sim, G.S.; Lee, B.C.; Cho, H.S.; Lee, J.W.; Kim, J.H.; Lee, D.H.; Kim, J.H.; Pyo, H.B.; Moon, D.C.; Oh, K.W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Tamogami, S.; Kodama, O.; Tsukiboshi, T. Synthesis of 7-methoxyapigeninidin and its fungicidal activity against Gloeocercospora sorghi. Biosci. Biotechnol. Biochem. 1996, 60, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sato, T.; Akimoto, N.; Yano, M.; Ito, A. Prevention of UVB-induced photoinflammation and photoaging by a polymethoxy flavonoid, nobiletin, in human keratinocytes in vivo and in vitro. Biochem. Pharmacol. 2004, 68, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation alters transforming growth factor β/smad pathway in human skin in vivo. J. Investig. Dermatol. 2002, 119, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Fu, Y.Y.; Chung, I.S.; Hahn, T.R.; Cho, M.H. Cytotoxic property of UV-induced rice phytoalexins to human colon carcinoma HCT-116 Cells. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 237–241. [Google Scholar] [CrossRef]
- Park, H.L.; Lee, S.W.; Jung, K.H.; Hahn, T.R.; Cho, M.H. Transcriptomic analysis of UV-treated rice leaves reveals UV-induced phytoalexin biosynthetic pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Han, J.; Lee, C.S.; Soo, B.H.; Lim, K.M.; Ha, H. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp. Dermatol. 2013, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kwak, H.B.; Kim, H.H.; Lee, Z.H.; Chung, D.K.; Baek, N.I.; Kim, J. Methanol extracts of Stewartia koreana inhibit cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) gene expression by blocking NF-κB transactivation in LPS-activated RAW 264.7 cells. Mol. Cells 2007, 23, 398–404. [Google Scholar] [PubMed]
- Baumann, B.; Weber, C.K.; Troppmair, J.; Whiteside, S.; Israel, A.; Rapp, U.R.; Wirth, T. Raf induces NF-κB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc. Natl. Acad. Sci. USA 2000, 97, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Lee, E.H.; Lee, T.H.; Cho, M.-H. The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging. Int. J. Mol. Sci. 2016, 17, 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091449
Jung H, Lee EH, Lee TH, Cho M-H. The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging. International Journal of Molecular Sciences. 2016; 17(9):1449. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091449
Chicago/Turabian StyleJung, Hana, Eunjoo H. Lee, Tae Hoon Lee, and Man-Ho Cho. 2016. "The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging" International Journal of Molecular Sciences 17, no. 9: 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091449




