Identification of Bioactivity, Volatile and Fatty Acid Profile in Supercritical Fluid Extracts of Mexican arnica
Abstract
:1. Introduction
2. Results and Discussion
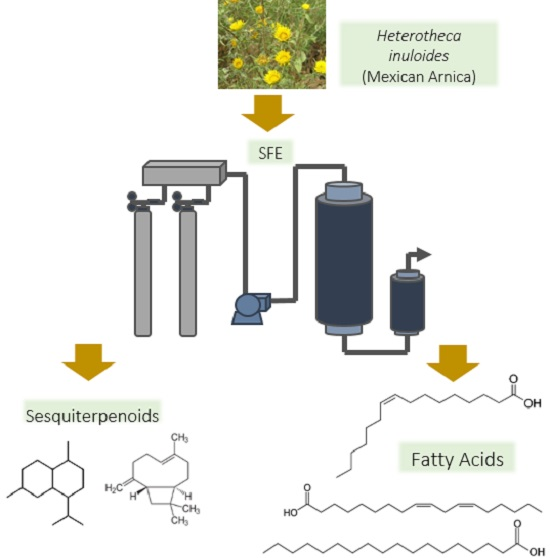
2.1. Extraction Yield
2.2. Gas Chromatography (GC) Analysis
2.3. Fatty Acid Profile
2.4. Antimicrobial Susceptibility Test
2.5. Antioxidant Capacity
3. Materials and Methods
3.1. Biological Material
3.2. SC-CO2 Extraction and Design of Experiments (DOE)
3.3. Gas Chromatography (GC) Analysis
3.4. Fatty Acid Determination
3.5. Antimicrobial Susceptibility Test
3.6. Antioxidant Capacity
3.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.6.2. Ferric Reducing Antioxidant Power (FRAP)
3.6.3. ABTS Antioxidant Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Camacho-Carranza, R.; Cárdenas-Rodríguez, N.; Huerta-Gertrudis, B.; Medina-Campos, O.N.; Mendoza-Cruz, M.; Delgado-Lamas, G.; Espinosa-Aguirre, J.J. Antioxidant activity of Heterotheca inuloides extracts and of some of its metabolites. Toxicology 2010, 276, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Calderón, G.; Rzedowski, J. Flora Fanerogámica del Valle de México; Instituto de Ecología: Valle de, Mexico, 2001. [Google Scholar]
- Kamatani, J.; Iwadate, T.; Tajima, R.; Kimoto, H.; Yamada, Y.; Masuoka, N.; Kubo, I.; Nihei, K.I. Stereochemical investigation and total synthesis of inuloidin, a biologically active sesquiterpenoid from Heterotheca inuloides. Tetrahedron 2014, 70, 3141–3145. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-κB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chávez, J.L.; Coballase-Urrutia, E.; Nieto-Camacho, A.; Delgado-Lamas, G. Antioxidant capacity of “Mexican arnica” Heterotheca inuloides cass natural products and some derivatives: Their anti-inflammatory evaluation and effect on C. elegans life span. Oxid. Med. Cell. Longev. 2015, 2015, 843237. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Parimelazhagan, T. Comparative evaluation of different extraction methods for antioxidant and anti-inflammatory properties from Osbeckia parvifolia Arn.—An in vitro approach. J. King Saud Univ. Sci. 2014, 26, 267–275. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.; López, V.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.; Cuéllar-Bermúdez, S.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical carbon dioxide and microwave-assisted extraction of functional lipophilic compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef] [PubMed]
- Raventos, M.; Duarte, S.; Alarcon, R. Application and possibilities of supercritical CO2 extraction in food processing industry: An overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Menaker, A.; Kravets, M.; Koel, M.; Orav, A. Identification and characterization of supercritical fluid extracts from herbs. C. R. Chim. 2004, 7, 629–633. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A.; Chaudhuri, S.K.; Sanchez, Y.; Ogura, T. Antimicrobial agents from Heterotheca inuloides. Planta Med. 1994, 60, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, M.C.; Bilia, A.R.; Casiraghi, A.; Cilurzo, F.; Minghetti, P.; Montanari, L.; Vincieri, F.F. Evaluation of skin permeability of sesquiterpenes of an innovative supercritical carbon dioxide Arnica extract by HPLC/DAD/MS. Pharmazie 2005, 60, 36–38. [Google Scholar] [PubMed]
- Garmus, T.T.; Paviani, L.C.; Queiroga, C.L.; Magalhães, P.M.; Cabral, F.A. Extraction of phenolic compounds from pitanga (Eugenia uniflora L.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2014, 86, 4–14. [Google Scholar] [CrossRef]
- Fornari, T.; Ruiz-Rodriguez, A.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Kinetic study of the supercritical CO2 extraction of different plants from Lamiaceae family. J. Supercrit. Fluids 2012, 64, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Delgado, G.; del Socorro Olivares, M.; Chávez, M.I.; Ramírez-Apan, T.; Linares, E.; Bye, R.; Espinosa-García, F.J. Antiinflammatory constituents from Heterotheca inuloides. J. Nat. Prod. 2001, 64, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Chaudhuri, S.K.; Kubo, Y.; Sanchez, Y.; Ogura, T.; Saito, T.; Ishikawa, H.; Haraguchi, H. Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med. 1996, 62, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chávez, J.L.; Coballase-Urrutia, E.; Sicilia-Argumedo, G.; Ramírez-Apan, T.; Delgado, G. Toxicological evaluation of the natural products and some semisynthetic derivatives of Heterotheca inuloides Cass (Asteraceae). J. Ethnopharmacol. 2015, 175, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Mijangos Ricárdez, O.F.; Ruiz-Jiménez, J.; Lagunez-Rivera, L.; Luque De Castro, M.D. Fast ultrasound-assisted extraction of polar (phenols) and nonpolar (lipids) fractions in Heterotheca inuloides Cass. Phytochem. Anal. 2011, 22, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Mazzi, G.; Vincieri, F.F. Development and stability of semisolid preparations based on a supercritical CO2 Arnica extract. J. Pharm. Biomed. Anal. 2006, 41, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.J.; Villada, H.S.; Carrera, J.E. Aplicaciones de los fluidos supercríticos en la agroindustria. Inf. Tecnol. 2007, 18, 53–66. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ishikawa, H.; Sanchez, Y.; Ogura, T.; Kubo, Y.; Kubo, I. Antioxidative constituents in Heterotheca inuloides. Bioorg. Med. Chem. 1997, 5, 865–871. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I.; Chaudhuri, S.K.; Kubo, Y.; Sánchez, Y.; Ogura, T. Flavonols from Heterotheca inuloides: Tyrosinase inhibitory activity and structural criteria. Bioorg. Med. Chem. 2000, 8, 1749–1755. [Google Scholar] [CrossRef]
- Haraguchi, H.; Saito, T.; Ishikawa, H.; Sanchez, Y.; Ogura, T.; Kubo, I. Inhibition of lipid peroxidation by sesquiterpenoid in Heterotheca inuloides. J. Pharm. Pharmacol. 1996, 48, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Huerta-Gertrudis, B.; Edna García-Cruz, M.; Ramírez-Morales, A.; Javier Sánchez-González, D.; María Martínez-Martínez, C.; Camacho-Carranza, R.; Javier Espinosa-Aguirre, J. Hepatoprotective effect of acetonic and methanolic extracts of Heterotheca inuloides against CCl4-induced toxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.; Reátegui, P.; Paula, A.; Barbero, G.F.; Rezende, C.A.; Martínez, J. The Journal of Supercritical Fluids Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar]




| Treatment | T (°C) | P (MPa) | EtOH+ (g/min) | Extracted Mass (g) | Yield (%) |
|---|---|---|---|---|---|
| 1 ab | 40 | 10 | 4 | 0.55 ± 0.03 | 5.54 ± 0.30 |
| 2 a | 60 | 10 | 8 | 0.71 ± 0.02 | 7.13 ± 0.27 |
| 3 b | 40 | 30 | 8 | 0.46 ± 0.08 | 4.62 ± 0.87 |
| 4 a | 60 | 30 | 4 | 0.67 ± 0.14 | 6.69 ± 1.44 |
| Parameter | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|
| Pressure | 8.20 | 1 | 8.20 | 4.10 | 0.077 |
| Temperature | 8.32 | 1 | 8.32 | 4.17 | 0.075 |
| Co-solvent | 1.66 | 1 | 1.66 | 0.83 | 0.388 |
| Residual | 15.97 | 8 | 1.99 |
| Compound | MW * (g/mol) | Relative Area (%) Per Treatment | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 4(3H)-Quinazolinone, 7-amino-3-ethyl– | 189.09 | - | - | - | 2.732 |
| 7-Hydroxycadalene | 214.13 | 5.05 a | - | 4.913 a | 4.969 a |
| Cadalene | 198.14 | 10.34 a | 8.755 b | 9.520 ab | 9.709 ab |
| Caryophyllene | 204.18 | 1.35 a | 1.084 a | 1.117 a | 1.087 a |
| Caryophyllene oxide | 220.18 | 4.08 a | 3.494 a | 3.441 a | 4.131 a |
| Docosane | 310.36 | - | 10.185 a | 8.926 a | - |
| Eicosane | 282.32 | 2.09 a | - | 3.363 a | 2.202 a |
| Heneicosane | 296.34 | - | 5.653 a | 2.703 b | - |
| Hexacosane | 366.42 | 19.53 a | 8.685 b | 17.616 a | 14.146 b |
| Nonacosane | 408.47 | 8.61 a | 8.770 a | - | - |
| Octadecane | 254.29 | - | 1.443 | - | - |
| Octadecane, 1-iodo– | 380.19 | - | - | 3.534 a | 4.668 a |
| Pentacosane | 352.40 | 2.67 a | 2.954 a | 1.701 a | 1.540 a |
| Spathulenol | 220.18 | - | - | - | 0.603 |
| δ-Cadinene | 204.18 | 2.11 a | 1.879 b | 1.904 b | 1.913 b |
| Fatty Acid | Fatty Acid Percentage (%) Per Treatment | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Lauric C12:0 | 0.5 ± 0.0 | - | - | - |
| Myristic C14:0 | 2.4 ± 0.0 | 2.4 ± 0.01 | 2.6 ± 0.01 | 2.4 ± 0.01 |
| Palmitic C16:0 | 20.6 ± 0.02 | 21.4 ± 0.07 | 22.2 ± 0.12 | 21.2 ± 0.06 |
| Palmitoleic C16:1 | 1.2 ± 0.01 | 1.0 ± 0.0 | 1.1 ± 0.01 | 1.5 ± 0.01 |
| Stearic C18:0 | 7.6 ± 0.01 | 7.3 ± 0.03 | 8.5 ± 0.04 | 7.5 ± 0.02 |
| Oleic C18:1ω9c | 5.0 ± 0.01 | 5.1 ± 0.01 | 5.4 ± 0.03 | 5.4 ± 0.0 |
| Linolelaidic C18:2ω6t | 3.8 ± 0.02 | 2.5 ± 0.01 | 3.3 ± 0.02 | 2.9 ± 0.02 |
| Linoleic C18:2ω6c | 13.6 ± 0.01 | 16.4 ± 0.06 | 16.1 ± 0.08 | 15.5 ± 0.05 |
| γ-Linolenic C18:3ω6 | 1.8 ± 0.0 | 1.6 ± 0.01 | 1.9 ± 0.01 | 1.7 ± 0.01 |
| α-Linolenic C18:3ω3 | 7.0 ± 0.0 | 7.6 ± 0.03 | 7.5 ± 0.04 | 7.3 ± 0.03 |
| Behenic C22:0 | 2.5 ± 0.0 | 2.3 ± 0.01 | 2.6 ± 0.01 | 2.4 ± 0.01 |
| Erucic, C22:1ω9 | 1.7 ± 0.0 | 3.3 ± 0.01 | 2.5 ± 0.01 | 2.2 ± 0.01 |
| Lignoceric, C24:0 | 4.2 ± 0.01 | 3.9 ± 0.02 | 4.7 ± 0.03 | 4.2 ± 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pérez, J.S.; Cuéllar-Bermúdez, S.P.; Arévalo-Gallegos, A.; Rodríguez-Rodríguez, J.; Iqbal, H.M.N.; Parra-Saldivar, R. Identification of Bioactivity, Volatile and Fatty Acid Profile in Supercritical Fluid Extracts of Mexican arnica. Int. J. Mol. Sci. 2016, 17, 1528. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091528
García-Pérez JS, Cuéllar-Bermúdez SP, Arévalo-Gallegos A, Rodríguez-Rodríguez J, Iqbal HMN, Parra-Saldivar R. Identification of Bioactivity, Volatile and Fatty Acid Profile in Supercritical Fluid Extracts of Mexican arnica. International Journal of Molecular Sciences. 2016; 17(9):1528. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091528
Chicago/Turabian StyleGarcía-Pérez, J. Saúl, Sara P. Cuéllar-Bermúdez, Alejandra Arévalo-Gallegos, José Rodríguez-Rodríguez, Hafiz M. N. Iqbal, and Roberto Parra-Saldivar. 2016. "Identification of Bioactivity, Volatile and Fatty Acid Profile in Supercritical Fluid Extracts of Mexican arnica" International Journal of Molecular Sciences 17, no. 9: 1528. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091528