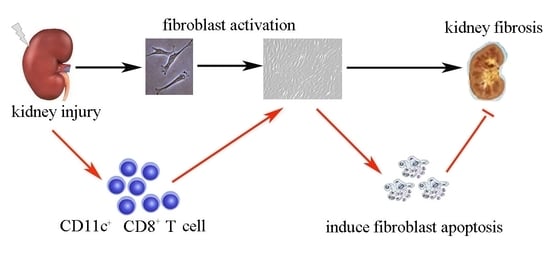
CD11c+ CD8+ T Cells Reduce Renal Fibrosis Following Ureteric Obstruction by Inducing Fibroblast Apoptosis
Abstract
:1. Introduction
2. Results
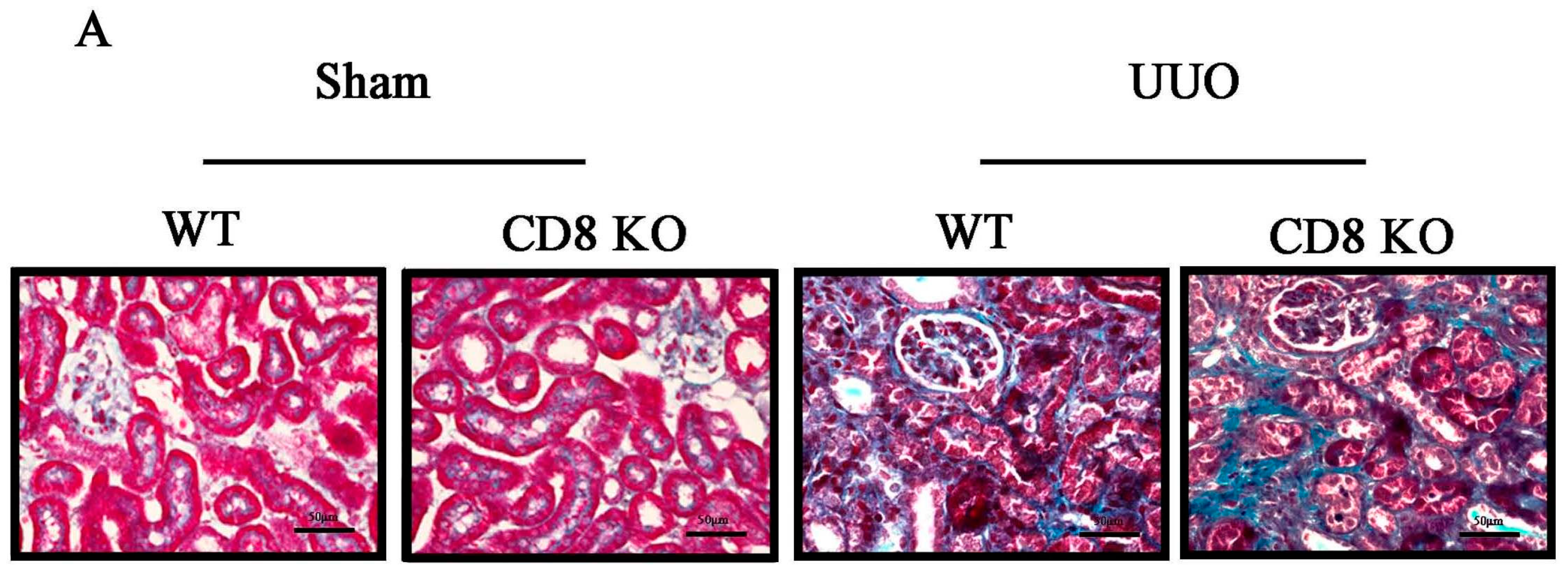
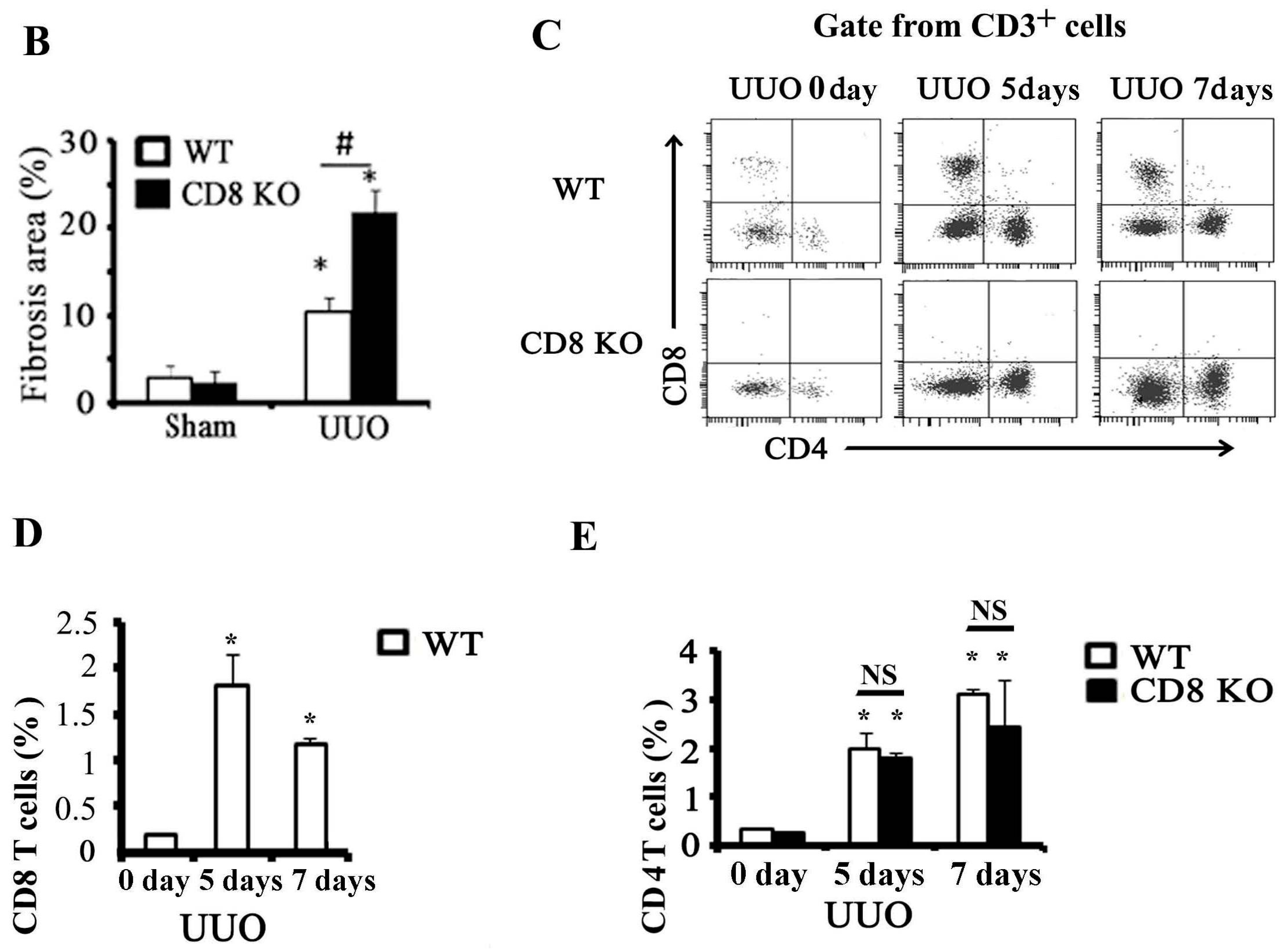
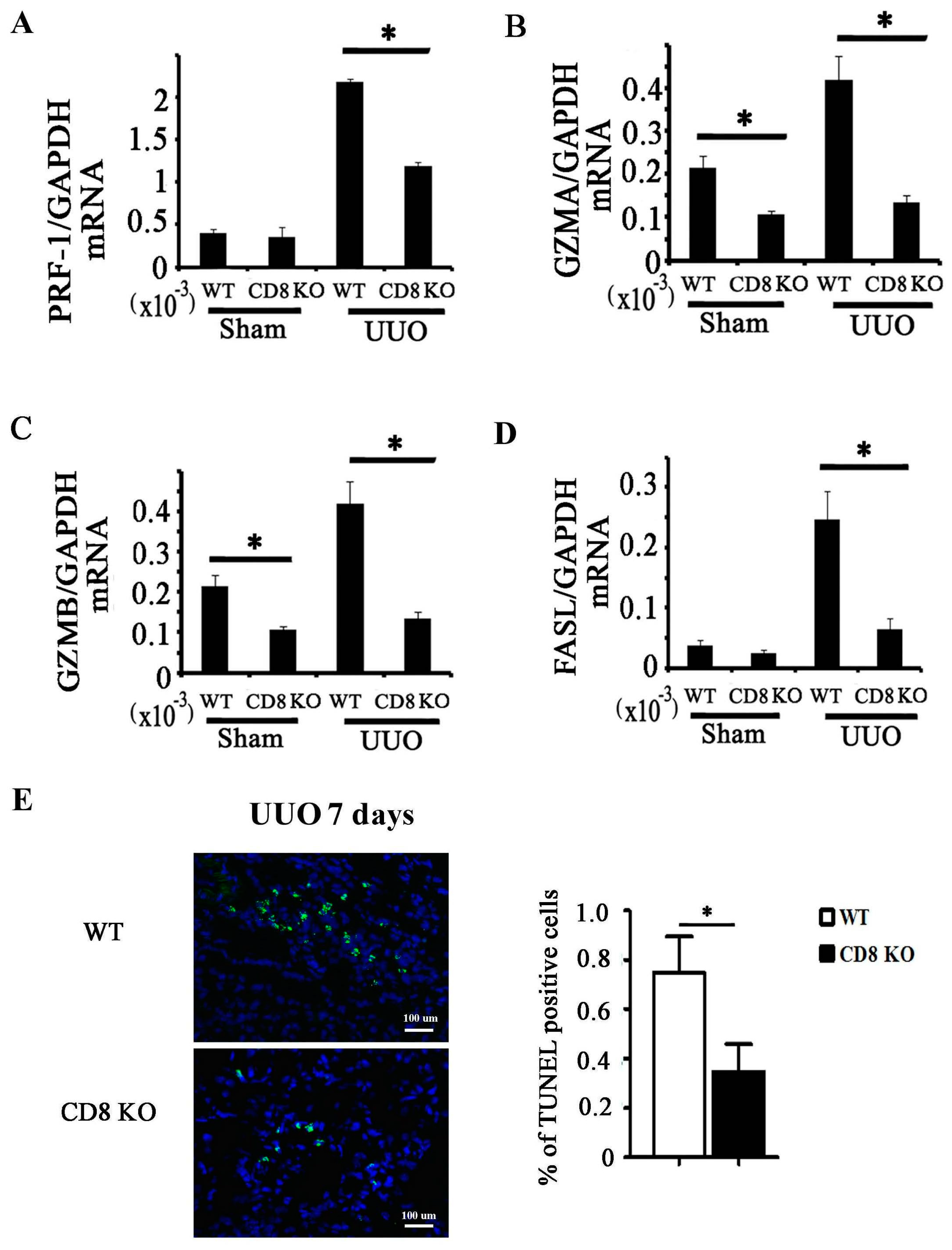
2.1. CD8 Deficiency Promotes Renal Fibrosis in Unilateral Ureteric Obstruction (UUO) Mouse Model
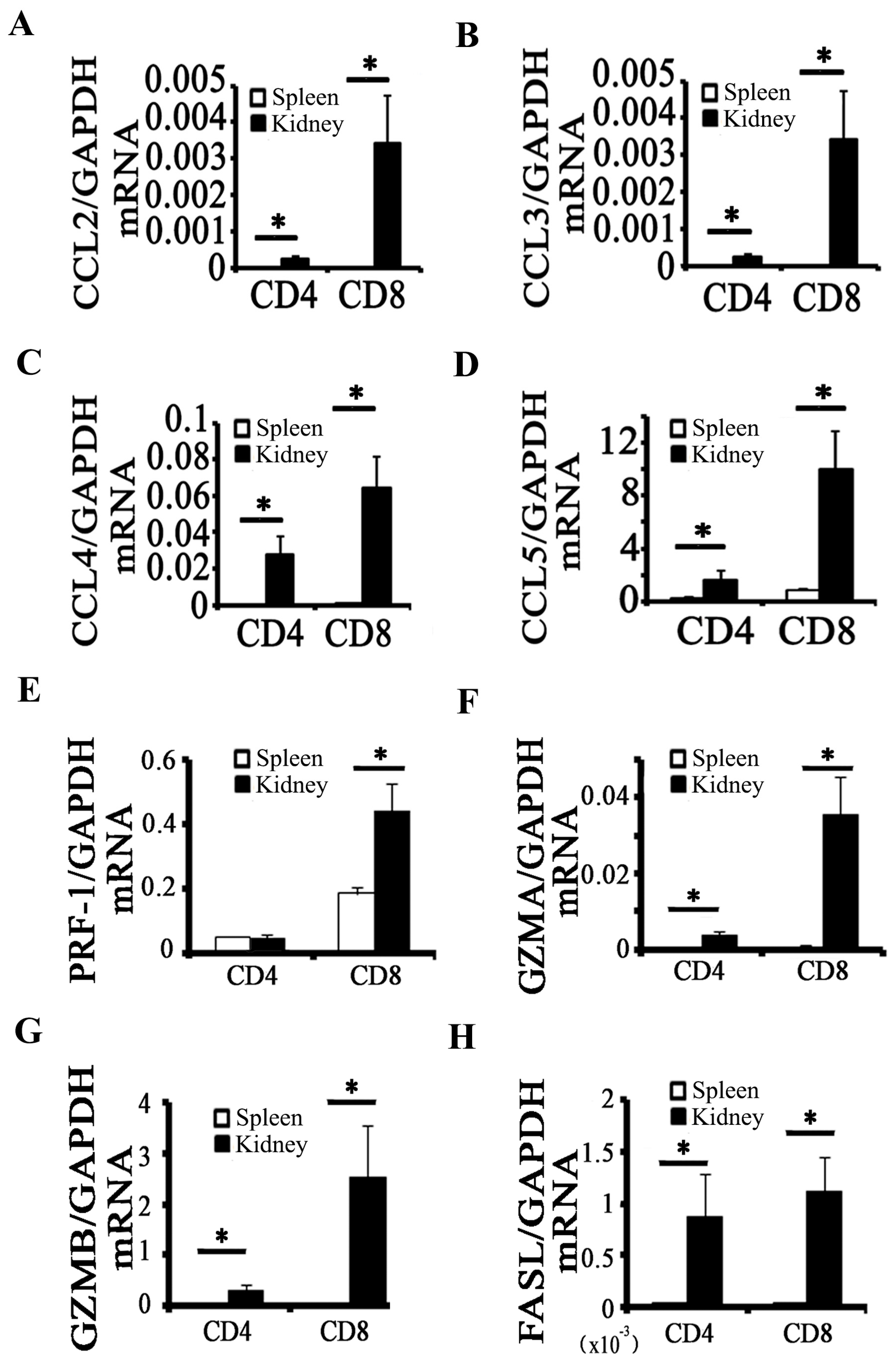
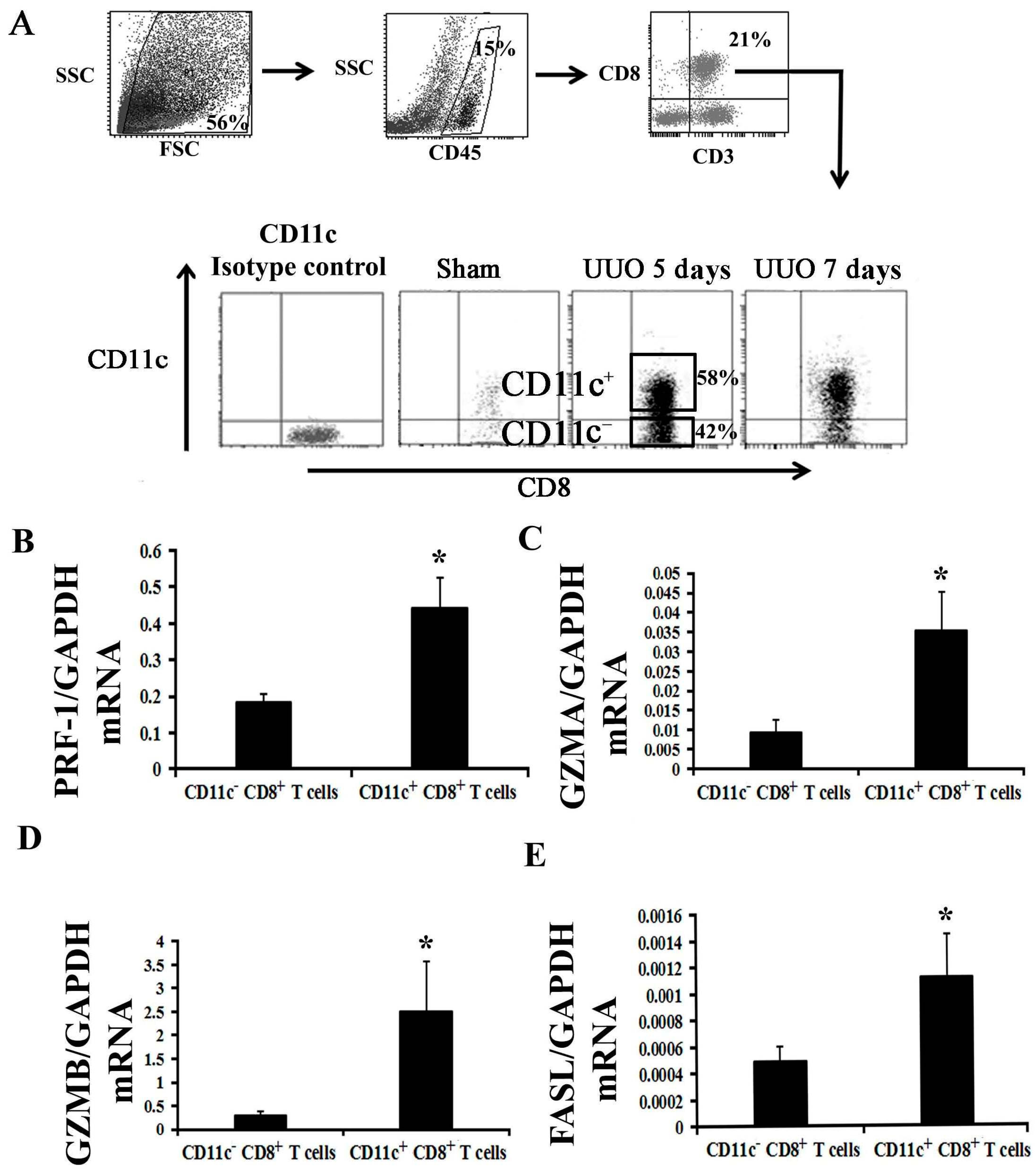
2.2. Activation of CD8+ T Cell Requires an Inflammatory Microenvironment
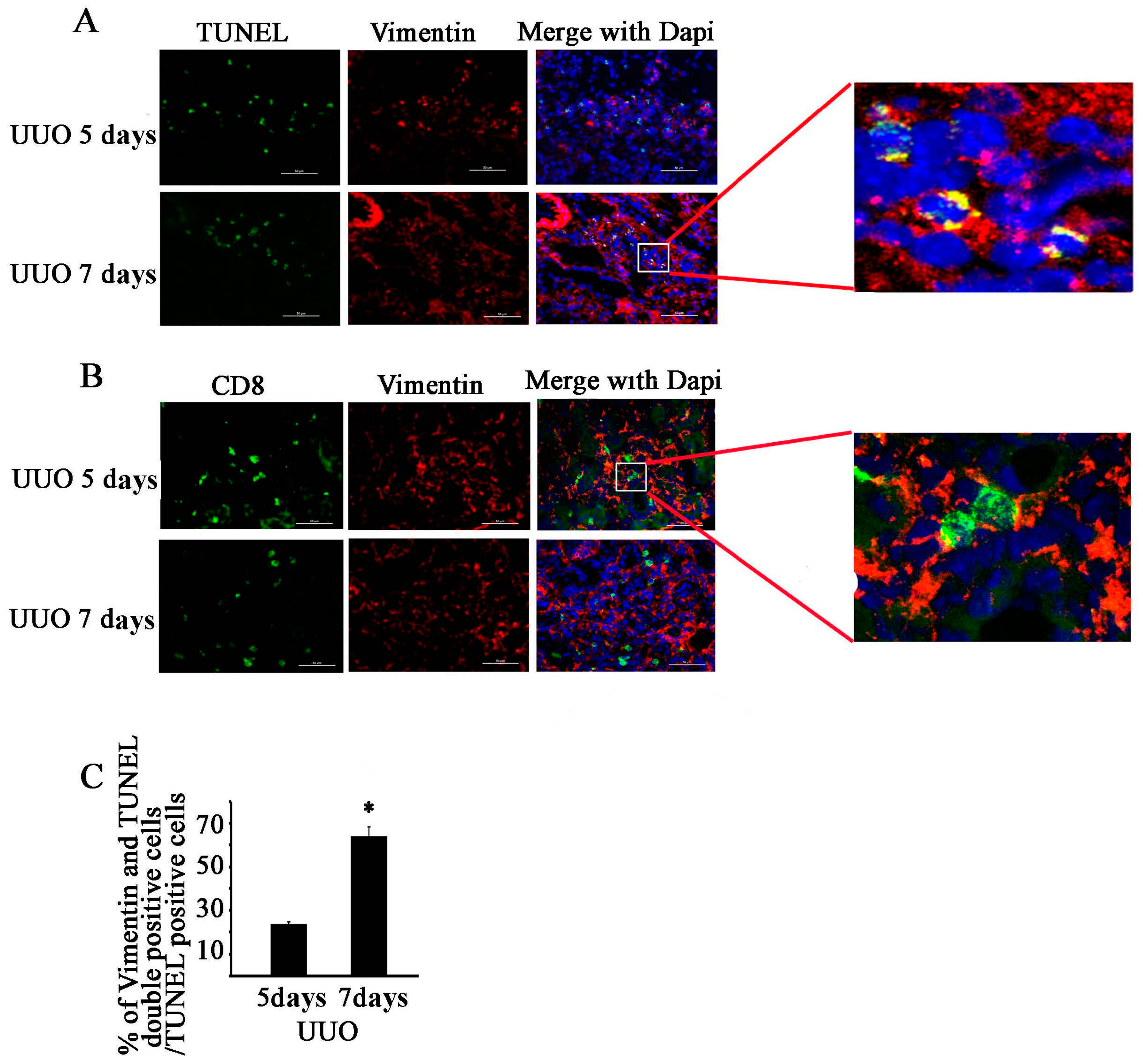
2.3. CD8 Deficiency Reduces Cytotoxicity and Cell Apoptosis in Obstructed Kidney
2.4. CD11c+ CD8+ T Cell Induces Fibroblast Death
3. Discussion
4. Materials and Methods
4.1. Animals and Surgery
4.2. Histology and Imaging
4.3. RT-PCR Analysis
4.4. Tissue Preparation, Fluorescence-Activated Cell Sorting, and Flow Cytometry
4.5. Fibroblast Isolation and Culture
4.6. Co-Culture Experiments
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harris, R.C.; Neilson, E.G. Toward a unified theory of renal progression. Annu. Rev. Med. 2006, 57, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.; Ali, S.; McDonnell, B.J.; Burt, A.D.; Kirby, J.A. Chronic renal allograft dysfunction: The role of T cell-mediated tubular epithelial to mesenchymal cell transition. J. Am. Soc. Nephrol. 2004, 15, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Vielhauer, V.; Frink, M.; Linde, Y.; Cohen, C.D.; Blattner, S.M.; Kretzler, M.; Strutz, F.; Mack, M.; Gröne, H.J.; et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J. Clin. Investig. 2002, 109, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Eis, V.; Luckow, B.; Vielhauer, V.; Siveke, J.T.; Linde, Y.; Segerer, S.; Lema, G.P.; Cohen, C.D.; Kretzler, M.; Mack, M.; et al. Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2004, 15, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Wada, T.; Furuichi, K.; Hashimoto, H.; Ishiwata, Y.; Asano, M.; Takeya, M.; Kuziel, W.A.; Matsushima, K.; Mukaida, N.; et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am. J. Pathol. 2004, 165, 237–246. [Google Scholar] [CrossRef]
- Tapmeier, T.T.; Fearn, A.; Brown, K.; Chowdhury, P.; Sacks, S.H.; Sheerin, N.S.; Wong, W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010, 78, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Vielhauer, V.; Anders, H.J.; Mack, M.; Cihak, J.; Strutz, F.; Stangassinger, M.; Luckow, B.; Gröne, H.J.; Schlöndorff, D. Obstructive nephropathy in the mouse: Progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J. Am. Soc. Nephrol. 2001, 12, 1173–1187. [Google Scholar] [PubMed]
- Dong, Y.; Yang, M.; Zhang, J.; Peng, X.; Cheng, J.; Cui, T.; Du, J. Depletion of CD8+ T cells exacerbates CD4+ T cell-induced monocyte-to-fibroblast transition in renal fibrosis. J. Immunol. 2016, 196, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Fung-Leung, W.P.; Schilham, M.W.; Rahemtulla, A.; Kündig, T.M.; Vollenweider, M.; Potter, J.; van Ewijk, M.; Mak, T.W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 1991, 65, 443–449. [Google Scholar] [CrossRef]
- Trapani, J.A.; Jans, D.A.; Jans, P.J.; Smyth, M.J.; Browne, K.A.; Sutton, V.R. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J. Boil. Chem. 1998, 273, 27934–27938. [Google Scholar] [CrossRef]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Golstein, P. The Fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. The origin of scar-forming kidney myofibroblasts. Nat. Med. 2013, 19, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, Y.; Gu, Y.; Liu, Y.; Jiang, Z.; Zhang, M.; Cao, X. CD11chigh CD8+ regulatory T cell feedback inhibits CD4 T cell immune response via Fas ligand-Fas pathway. J. Immunol. 2013, 190, 6145–6154. [Google Scholar] [CrossRef] [PubMed]
- Vernon, M.A.; Mylonas, K.J.; Hughes, J. Macrophages and renal fibrosis. Semin. Nephrol. 2010, 30, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.W.; Gadek, J.E.; Lawley, T.J.; Ronald, G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J. Clin. Investig. 1981, 68, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through Facilitating MCP-1 secretion and Gr1high macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, C.T.; Ma, T.T.; Li, Z.Y.; Zhou, L.N.; Mu, B.; Leng, F.; Shi, H.S.; Li, Y.O.; Wei, Y.Q. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010, 101, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.; D’Aiuto, E.; Vettori, S.; Cuoppolo, P.; Abbate, G.; Valentini, G. Peripheral T cells from patients with early systemic sclerosis kill autologous fibroblasts in co-culture: Is T cell response aimed to play a protective role? Rheumatology 2010, 49, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Bangen, J.M.; Govaere, O.; Zimmermann, H.W.; Gassler, G.; Huss, S.; Liedtke, C.; Prinz, I.; Lira, S.A.; Luedde, T.; et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 2014, 59, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Wang, Y.; Woodard, L.E.; Wilson, M.H.; Sharma, R.; Awasthi, Y.C.; Du, J.; Mitch, W.E.; Cheng, J. Loss of glutathione S-transferase A4 accelerates obstruction-induced tubule damage and renal fibrosis. J. Pathol. 2012, 228, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]

| mRNA | Forward | Reverse |
|---|---|---|
| PRF-1 | 5′-AAAAACTCCCTAATGAGAGACGC-3′ | 5′-ACACGCCAGTCGTTATTGATATT-3′ |
| GZMA | 5′-TGCTGCCCACTGTAACGTG-3′ | 5′-GGTAGGTGAAGGATAGCCACAT-3′ |
| GZMB | 5′-CCACTCTCGACCCTACATGG-3′ | 5′-GGCCCCCAAAGTGACATTTATT-3′ |
| FasL | 5′-TCCGTGAGTTCACCAACCAAA-3′ | 5′-GGGGGTTCCCTGTTAAATGGG-3′ |
| CCL2 | 5′-GTCTGTGCTGACCCCAAGAAG-3′ | 5′-TGGTTCCGATCCAGGTTTTTA-3′ |
| CCL3 | 5′-TTCTCTGTACCATGACACTCTGC-3′ | 5′-CGTGGAATCTTCCGGCTGTAG-3′ |
| CCL4 | 5′-TTCCTGCTGTTTCTCTTACACCT-3′ | 5′-CTGTCTGCCTCTTTTGGTCAG-3′ |
| CCL5 | 5′-AGATCTCTGCAGCTGCCCTCA-5′ | 5′-GGAGCACTTGCTGCTGGTGTAG-3′ |
| GAPDH | 5′-TGCCCCCATGTTTGTGATG-3′ | 5′-TGTGGTCATGAGCCCTTCC-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, J.; Bai, Y.; Li, J.; Li, L.; Dong, Y. CD11c+ CD8+ T Cells Reduce Renal Fibrosis Following Ureteric Obstruction by Inducing Fibroblast Apoptosis. Int. J. Mol. Sci. 2017, 18, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010001
Wang H, Wang J, Bai Y, Li J, Li L, Dong Y. CD11c+ CD8+ T Cells Reduce Renal Fibrosis Following Ureteric Obstruction by Inducing Fibroblast Apoptosis. International Journal of Molecular Sciences. 2017; 18(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010001
Chicago/Turabian StyleWang, Haidong, Juan Wang, Yun Bai, Jinwei Li, Lixin Li, and Yanjun Dong. 2017. "CD11c+ CD8+ T Cells Reduce Renal Fibrosis Following Ureteric Obstruction by Inducing Fibroblast Apoptosis" International Journal of Molecular Sciences 18, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010001