Identification of Proteins Interacting with Cytoplasmic High-Mobility Group Box 1 during the Hepatocellular Response to Ischemia Reperfusion Injury
Abstract
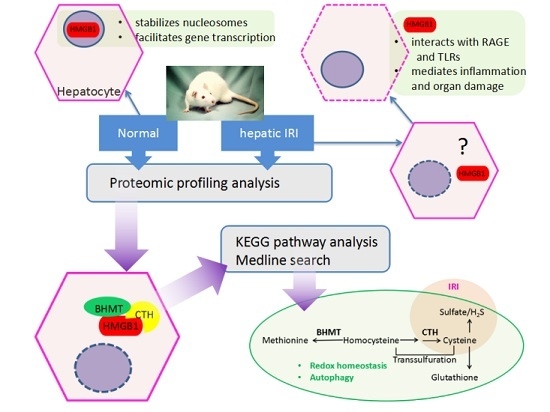
:1. Introduction
2. Results
2.1. Enrichment of Cytoplasmic High-Mobility Group Box 1 (HMGB1)-Binding Protein Complex from Warm Ischemia Reperfusion (WI/R) Liver Tissue
2.2. Identification and Verification of Binding Proteins of Cytoplasmic HMGB1 in WI/R Liver Tissue
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Animals and Selective Liver Ischemia
4.3. Protein Extraction and Co-Immunoprecipitation
4.4. Two-Dimensional Electrophoresis and Silver Staining
4.5. Mass Spectrometry Detection
4.6. Western Blotting
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IRI | Ischemia reperfusion injury |
| WI/R | Warm ischemia reperfusion |
| WB | Western blotting |
| co-IP | Co-immunoprecipitation |
| 2DE/MS | 2-dimensional electrophoresis and mass spectrometry |
| BHMT | Betaine–homocysteine S-methyltransferase 1 |
| CTH | Cystathionine γ-lyase |
References
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, G.; Freeze, H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 2009, 11, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Dirsch, O.; Fang, H.; Sun, J.; Jin, H.; Dong, W.; Dahmen, U. HMGB1 in ischemic and non-ischemic liver after selective warm ischemia/reperfusion in rat. Histochem. Cell Biol. 2011, 135, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Fang, H.; Dirsch, O.; Jin, H.; Dahmen, U. Oxidation of HMGB1 causes attenuation of its pro-inflammatory activity and occurs during liver ischemia and reperfusion. PLoS ONE 2012, 7, e35379. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, M.; Froese, S.; Dai, F.F.; Robitaille, M.; Bhattacharjee, A.; Huang, X.; Jia, W.; Angers, S.; Wheeler, M.B.; et al. The Identification of Novel Protein-Protein Interactions in Liver that Affect Glucagon Receptor Activity. PLoS ONE 2015, 10, e0129226. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, P.; Williams, J.S.; Kao, T.H. Identification of the self-incompatibility locus F-box protein-containing complex in Petunia inflata. Plant Reprod. 2014, 27, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Langlais, P.; Luo, M.; Mapes, R.; Lefort, N.; Chen, S.C.; Mandarino, L.J.; Yi, Z. Label-free proteomic identification of endogenous, insulin-stimulated interaction partners of insulin receptor substrate-1. J. Am. Soc. Mass Spectrom. 2011, 22, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Livesey, K.M.; Kang, R.; Zeh, H.J., III; Lotze, M.T.; Tang, D. Direct molecular interactions between HMGB1 and TP53 in colorectal cancer. Autophagy 2012, 8, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.; Tyagi, S.C. Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int. J. Mol. Sci. 2013, 14, 15074–15091. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Akahoshi, N.; Kamata, S.; Hagiya, Y.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Takano, N.; Mori, M.; Ishizaki, Y.; et al. Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine γ-lyase, an animal model of cystathioninuria. Free Radic. Biol. Med. 2012, 52, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Vowinckel, J.; Keller, M.A.; Ralser, M. Methionine Metabolism Alters Oxidative Stress Resistance via the Pentose Phosphate Pathway. Antioxid. Redox Signal. 2016, 24, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Caballero, V.J.; Mendieta, J.R.; Lombardo, D.; Saceda, M.; Ferragut, J.A.; Conde, R.D.; Giudici, A.M. Liver damage and caspase-dependent apoptosis is related to protein malnutrition in mice: Effect of methionine. Acta Histochem. 2015, 117, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Kevil, C.G. Nitric Oxide and Hydrogen Sulfide Regulation of Ischemic Vascular Remodeling. Microcirculation 2016, 23, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.; Webb, G.D.; Bian, J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef] [PubMed]
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.O.; Zancanela, V.; Gasparin, F.R.; Constantin, J.; Oliveira Neto, A.R. Effects of methionine supplementation on the redox state of acute heat stress-exposed quails. J. Anim. Sci. 2014, 92, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. Neurodegeneration in Huntington’s disease involves loss of cystathionine γ-lyase. Cell Cycle 2014, 13, 2491–2493. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Xu, R.; Vandiver, M.S.; Cha, J.Y.; Snowman, A.M.; Snyder, S.H. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature 2014, 509, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Sutter, B.M.; Wu, X.; Laxman, S.; Tu, B.P. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell 2013, 154, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.C.; García, I.; Gotor, C. L-Cysteine Desulfhydrase 1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal. Behav. 2013, 8, 4621–4634. [Google Scholar] [CrossRef] [PubMed]
- Talaei, F.; van Praag, V.M.; Henning, R.H. Hydrogen sulfide restores a normal morphological phenotype in Werner syndrome fibroblasts, attenuates oxidative damage and modulates mTOR pathway. Pharmacol. Res. 2013, 74, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Garcia, I.; Moreno, I.; Perez-Perez, M.E.; Crespo, J.L.; Romero, L.C.; Gotor, C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell 2012, 24, 4621–4634. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine γ-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Messer, J.S.; Wang, Y.; Lin, F.; Cham, C.M.; Chang, J.; Billiar, T.R.; Lotze, M.T.; Boone, D.L.; Chang, E.B. Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J. Clin. Investig. 2015, 125, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011, 30, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Huebener, P.; Gwak, G.Y.; Pradere, J.P.; Quinzii, C.M.; Friedman, R.; Lin, C.S.; Trent, C.M.; Mederacke, I.; Zhao, E.; Dapito, D.H.; et al. High-mobility group box 1 is dispensable for autophagy, mitochondrial quality control, and organ function in vivo. Cell Metab. 2014, 19, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Takizawa, S.; van Ypersele, D.S. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: Novel therapeutic targets. Am. J. Physiol. Cell Physiol. 2011, 300, C226–C231. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.X.; et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Ishii, I.; Shinmura, K.; Tamaki, K.; Hishiki, T.; Akahoshi, N.; Ida, T.; Nakanishi, T.; Kamata, S.; Kumagai, Y.; et al. Hyperhomocysteinemia abrogates fasting-induced cardioprotection against ischemia/reperfusion by limiting bioavailability of hydrogen sulfide anions. J. Mol. Med. 2015, 93, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Marko, L.; Szijarto, I.A.; Filipovic, M.R.; Kassmann, M.; Balogh, A.; Park, J.K.; Przybyl, L.; N’diaye, G.; Kramer, S.; Anders, J.; et al. Role of Cystathionine γ-Lyase in Immediate Renal Impairment and Inflammatory Response in Acute Ischemic Kidney Injury. Sci. Rep. 2016, 6, 27517. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Detergents: Triton X-100, Tween-20, and more. Mater. Methods 2013, 3, 163. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Krüger, T.; Knupfer, U.; Kasper, L.; Wielsch, N.; Hube, B.; Kortgen, A.; Bauer, M.; Giamarellos-Bourboulis, E.J.; Dimopoulos, G.; et al. Immunoproteomic Analysis of Antibody Responses to Extracellular Proteins of Candida albicans Revealing the Importance of Glycosylation for Antigen Recognition. J. Proteome Res. 2016, 15, 2394–2406. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, J.A.; Csordas, A.; del Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, D447–D456. [Google Scholar] [CrossRef] [PubMed]





| Accession 1 | Protein | Region No. | Score | Coverage | No. of Peptides | MW(kDa) 2 | calc. pI 3 | Biological Function 4 |
|---|---|---|---|---|---|---|---|---|
| 13540663 | Betaine—homocysteine S-methyltransferase 1 | 1 | 566.87 | 10.57 | 6 | 44.9 | 7.91 | Metabolic pathways; Cysteine and methionine metabolism |
| 13540663 | Betaine—homocysteine S-methyltransferase 1 | 2 | 566.74 | 16.46 | 10 | 44.9 | 7.91 | |
| 56030 | Cystathionine γ-lyase | 2 | 80.63 | 10.44 | 4 | 39.7 | 8.03 | Metabolic pathways; Cysteine and methionine metabolism; Sulfide production |
| 25453414 | Argininosuccinate synthase | 2 | 291.93 | 17.23 | 10 | 46.5 | 7.78 | Alanine, aspartate and glutamate metabolism; Arginine biosynthesis; Biosynthesis of amino acids; Urea cycle |
| 1374715 | ATP synthase β subunit | 3 | 360.79 | 8.42 | 4 | 51.2 | 5.02 | Oxidative phosphorylation; Metabolic pathways |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wei, W.; Dirsch, O.; Krüger, T.; Kan, C.; Xie, C.; Kniemeyer, O.; Fang, H.; Settmacher, U.; Dahmen, U. Identification of Proteins Interacting with Cytoplasmic High-Mobility Group Box 1 during the Hepatocellular Response to Ischemia Reperfusion Injury. Int. J. Mol. Sci. 2017, 18, 167. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010167
Zhang T, Wei W, Dirsch O, Krüger T, Kan C, Xie C, Kniemeyer O, Fang H, Settmacher U, Dahmen U. Identification of Proteins Interacting with Cytoplasmic High-Mobility Group Box 1 during the Hepatocellular Response to Ischemia Reperfusion Injury. International Journal of Molecular Sciences. 2017; 18(1):167. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010167
Chicago/Turabian StyleZhang, Tianjiao, Weiwei Wei, Olaf Dirsch, Thomas Krüger, Chunyi Kan, Chichi Xie, Olaf Kniemeyer, Haoshu Fang, Utz Settmacher, and Uta Dahmen. 2017. "Identification of Proteins Interacting with Cytoplasmic High-Mobility Group Box 1 during the Hepatocellular Response to Ischemia Reperfusion Injury" International Journal of Molecular Sciences 18, no. 1: 167. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010167