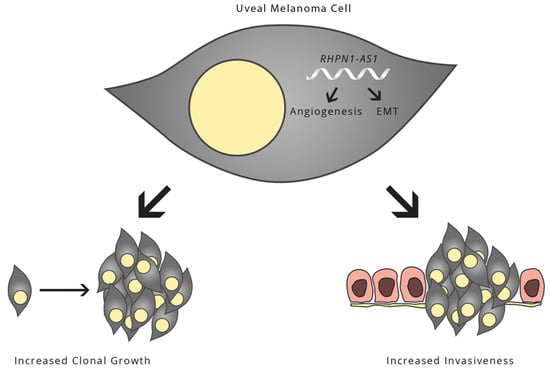
The Long Non-Coding RNA RHPN1-AS1 Promotes Uveal Melanoma Progression
Abstract
:1. Introduction
2. Results
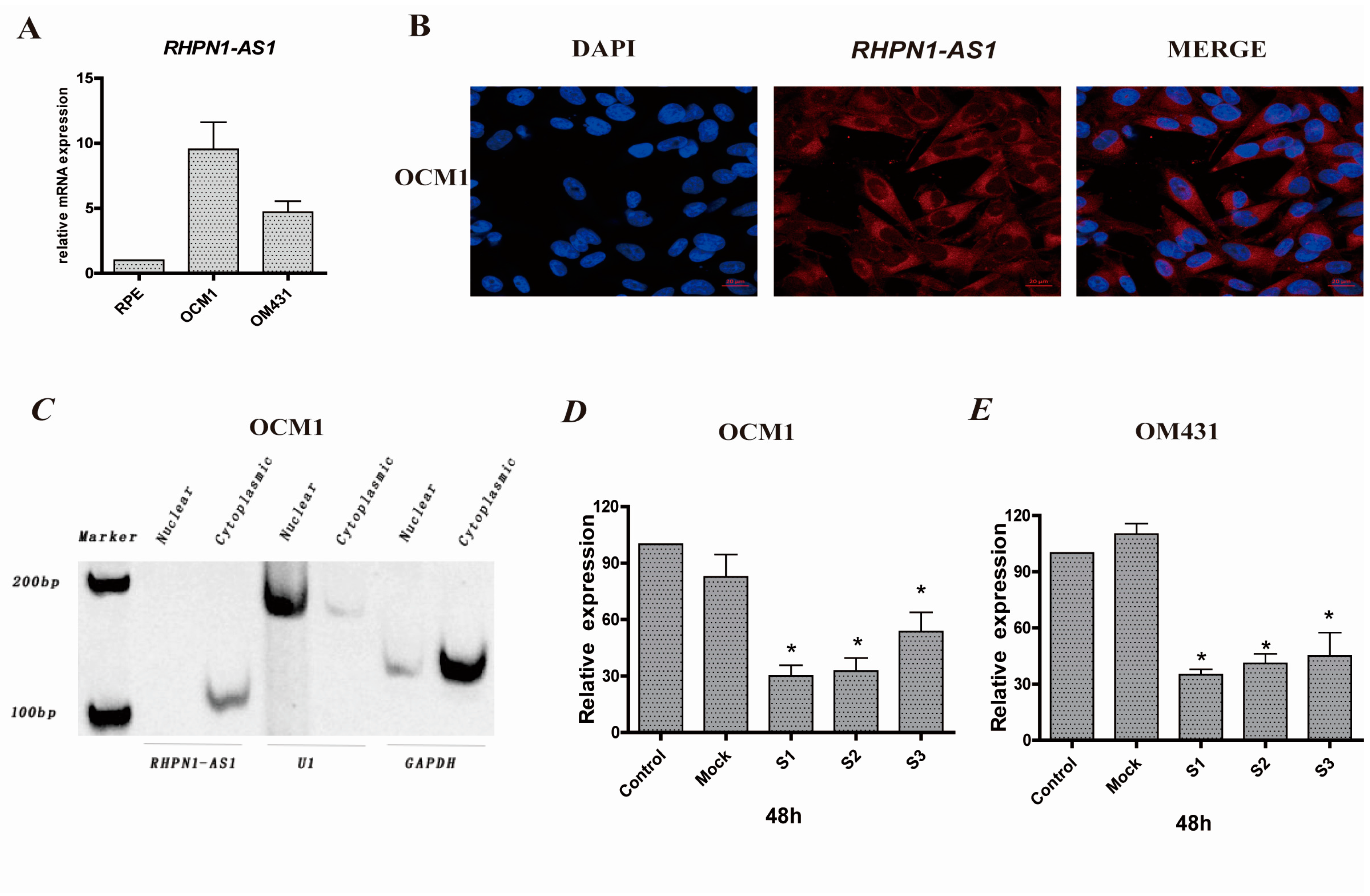
2.1. RHPN1-AS1 Is a Cytoplasmic lncRNA That Is Upregulated in Uveal Melanoma (UM)
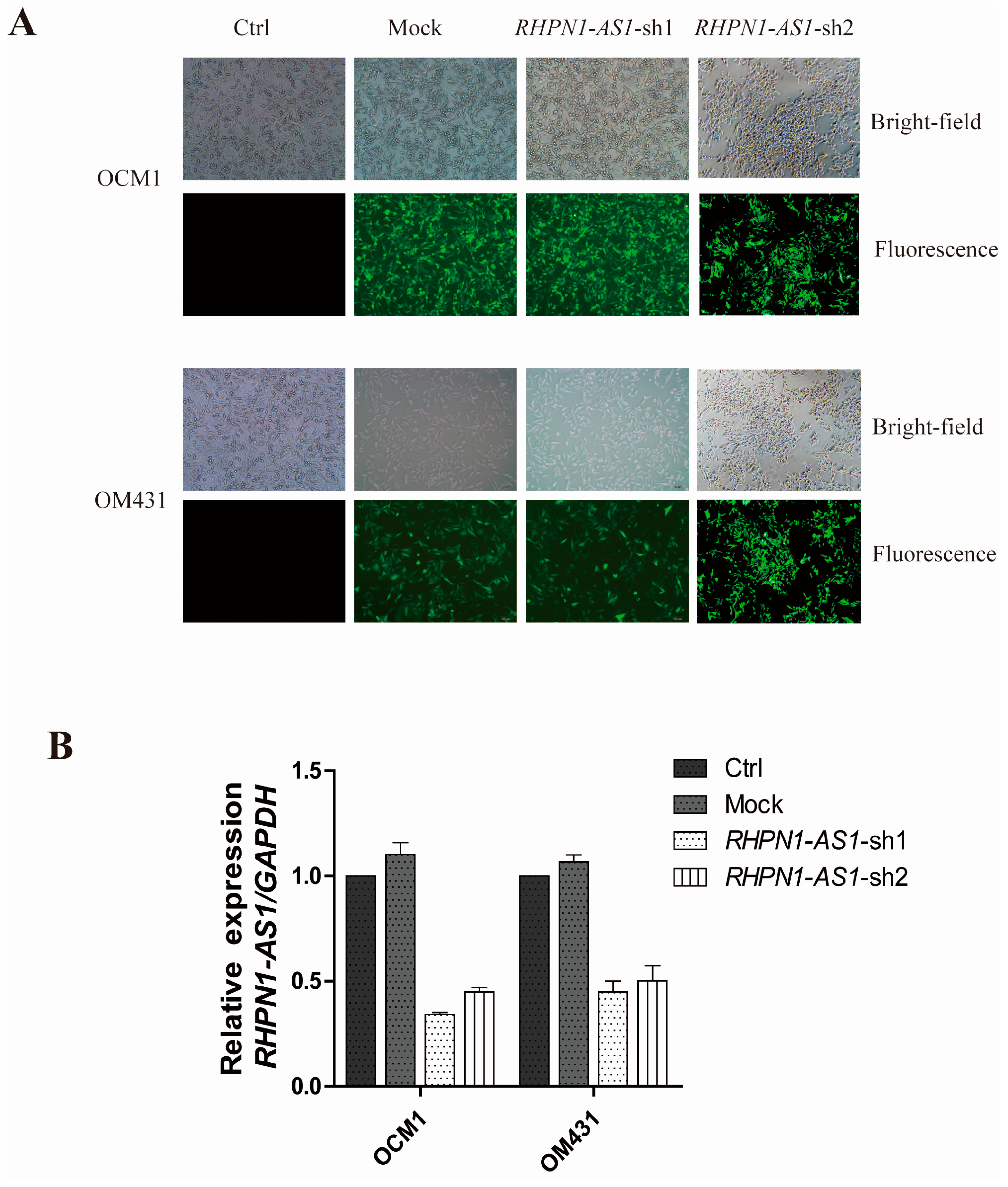
2.2. RHPN1-AS1 Knockdown Cell Lines
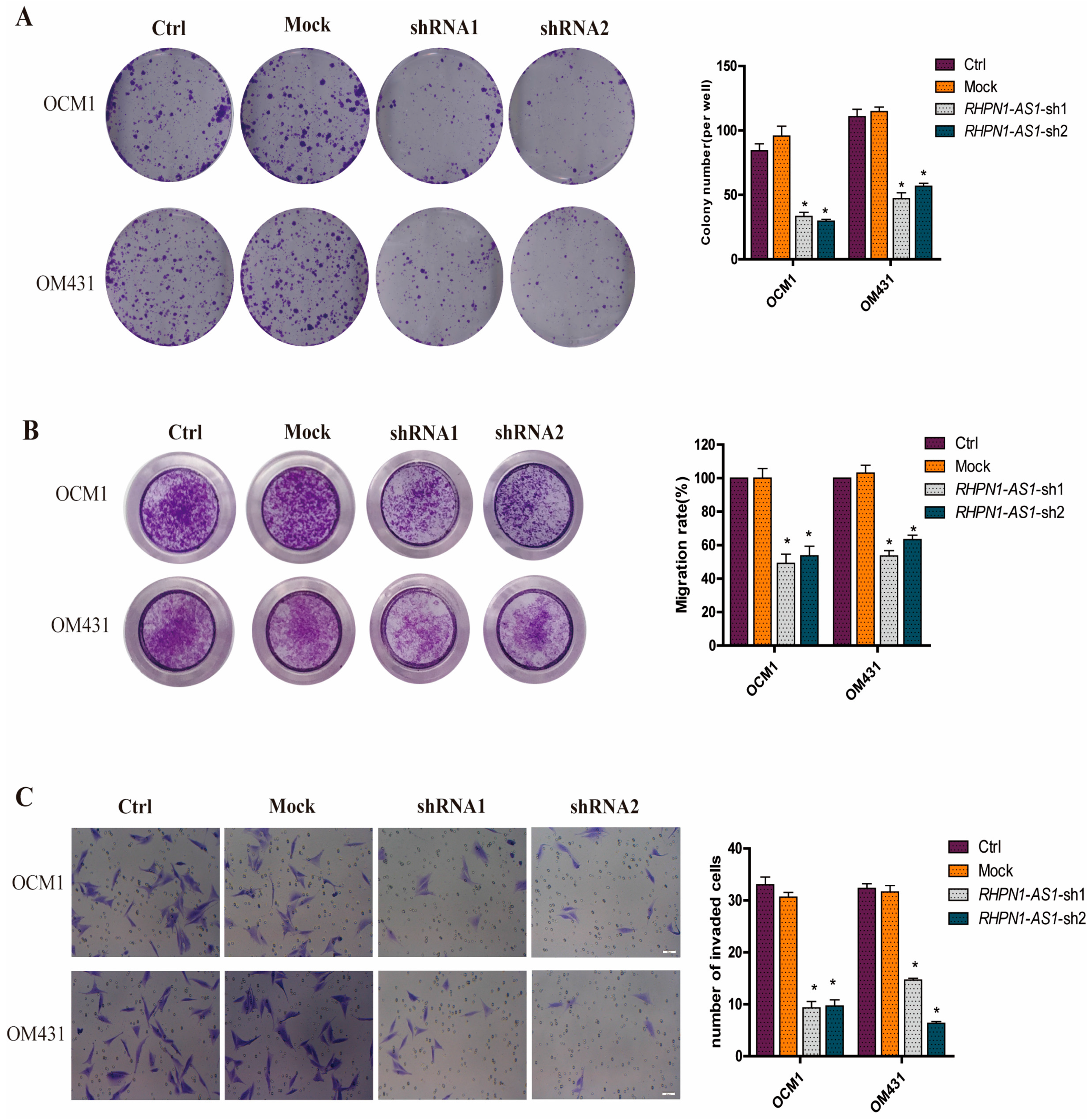
2.3. Down-Regulation of RHPN1-AS1 Inhibited Cell Proliferation, Migrationand Invasion In Vitro
2.4. Down-Regulation of RHPN1-AS1 Decreased Xenograft Growth In Vivo
2.5. Gene Expression Profile Analysis Revealed That Angiogenesis and Multiple Pathways May Be Downstream Targets Affected by RHPN1-AS1
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Short Interfering RNAs (siRNAs) in UM Cells
4.2. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assays
4.3. FluorescenceIn Situ Hybridization
4.4. Short Hairpin (sh) RNA-Expressing Plasmid Construction, Lentivirus Packaging, Cloning and Stable Transfection
4.5. Cell Migration Assay
4.6. Cell Invasion Assay
4.7. Colony Formation Assay
4.8. Tumor Xenograft Model in Nude Mice
4.9. Nuclear/Cytoplasmic RNA Fractionation
4.10. DNA Microarray Analysis and Data Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bol, K.F.; Mensink, H.W.; Aarntzen, E.H.; Schreibelt, G.; Keunen, J.E.; Coulie, P.G.; de Klein, A.; Punt, C.J.; Paridaens, D.; Figdor, C.G.; et al. Long Overall Survival after Dendritic Cell Vaccination in Metastatic Uveal Melanoma Patients. Am. J. Ophthalmol. 2014, 158, 939–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietschel, P.; Panageas, K.S.; Hanlon, C.; Patel, A.; Abramson, D.H.; Chapman, P.B. Variates of Survival in Metastatic Uveal Melanoma. J. Clin. Oncol. 2005, 23, 8076–8080. [Google Scholar] [CrossRef] [PubMed]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of Metastatic Disease after Enrollment in the Coms Trials for Treatment of Choroidal Melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar] [PubMed]
- Buzzacco, D.M.; Abdel-Rahman, M.H.; Park, S.; Davidorf, F.; Olencki, T.; Cebulla, C.M. Long-Term Survivors with Metastatic Uveal Melanoma. Open Ophthalmol. J. 2012, 6, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Furney, S.J.; Pedersen, M.; Gentien, D.; Dumont, A.G.; Rapinat, A.; Desjardins, L.; Turajlic, S.; Piperno-Neumann, S.; de la Grange, P.; Roman-Roman, S.; et al. SF3B1 Mutations Are Associated with Alternative Splicing in Uveal Melanoma. Cancer Discov. 2013, 3, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in Uveal Melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent Mutation of BAP1 in Metastasizing Uveal Melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, N.; Glavac, D. Long Non-Coding RNA in Cancer. Int. J. Mol. Sci. 2013, 14, 4655–4669. [Google Scholar] [CrossRef] [PubMed]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of Transcription in Human Cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin Signature Reveals over a Thousand Highly Conserved Large Non-Coding RNAs in Mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Diederichs, S. The Hallmarks of Cancer: A Long Non-Coding RNA Point of View. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xing, Y.; Wen, X.; Jia, R.; Ni, H.; He, J.; Ding, X.; Pan, H.; Qian, G.; Ge, S.; et al. Long Non-Coding RNA Ror Decoys Gene-Specific Histone Methylation to Promote Tumorigenesis. Genome Biol. 2015, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Horie-Inoue, K.; Katayama, S.; Suzuki, T.; Tsutsumi, S.; Ikeda, K.; Urano, T.; Fujimura, T.; Takagi, K.; Takahashi, S.; et al. Androgen-Responsive Long Noncoding RNACTBP1-as Promotes Prostate Cancer. EMBO J. 2013, 32, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, M.; Zhu, Y.N.; Xia, R.; Liu, Y.W.; Ding, J.; Ma, H.W.; He, X.Z.; Zhang, Z.H.; Liu, Z.J.; et al. Long Noncoding RNA HOXA-AS2 Promotes Gastric Cancer Proliferation by Epigenetically Silencing P21/PLK3/DDIT3 Expression. Oncotarget 2015, 6, 33587–33601. [Google Scholar] [PubMed]
- Calin, G.A.; Liu, C.G.; Ferracin, M.; Hyslop, T.; Spizzo, R.; Sevignani, C.; Fabbri, M.; Cimmino, A.; Lee, E.J.; Wojcik, S.E.; et al. Ultraconserved Regions Encoding ncRNAs Are Altered in Human Leukemias and Carcinomas. Cancer Cell 2007, 12, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Leung, E.Y.; Baguley, B.C.; Finlay, G.J.; Askarian-Amiri, M.E. Epigenetic Regulation in Human Melanoma: Past and Future. Epigenetics 2015, 10, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Dono, M.; Angelini, G.; Cecconi, M.; Amaro, A.; Esposito, A.I.; Mirisola, V.; Maric, I.; Lanza, F.; Nasciuti, F.; Viaggi, S.; et al. Mutation Frequencies of GNAQ, GNA11, BAP1, SF3B1, EIF1AX and TERT in Uveal Melanoma: Detection of an Activating Mutation in the TERT Gene Promoter in a Single Case of Uveal Melanoma. Br. J. Cancer 2014, 110, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T. Epigenetic Regulation by Long Noncoding Rnas. Science 2012, 338, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Iyer, M.K.; Balbin, O.A.; Dhanasekaran, S.M.; Cao, Q.; Brenner, J.C.; Laxman, B.; Asangani, I.A.; Grasso, C.S.; Kominsky, H.D.; et al. Transcriptome Sequencing across a Prostate Cancer Cohort Identifies PCAT-1, an Unannotated LincRNA Implicated in Disease Progression. Nat. Biotechnol. 2011, 29, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, T.; Lian, Y.; Zhang, G.; Garen, A.; Song, X. Role of Human Noncoding RNAs in the Control of Tumorigenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12956–12961. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Pilpel, Y. Genome-Wide Natural Antisense Transcription: Coupling Its Regulation to Its Different Regulatory Mechanisms. EMBO Rep. 2006, 7, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Mattick, J.S. Structure and Function of Long Noncoding RNAs in Epigenetic Regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Moran, V.A.; Perera, R.J.; Khalil, A.M. Emerging Functional and Mechanistic Paradigms of Mammalian Long Non-Coding RNAs. Nucleic Acids Res. 2012, 40, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Mechanisms of Tgfβ-Induced Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.L.; Tuzova, A.V.; Bolton, E.M.; Lynch, T.H.; Perry, A.S. Long Noncoding RNAs and Prostate Carcinogenesis: The Missing “Linc”? Trends Mol. Med. 2014, 20, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Nakagawa, T.; Kitagawa, K.; Suzuki, S.; Liu, N.; Kitagawa, M.; Xiong, Y. Long Non-Coding RNA ANRIL Is Required for the PRC2 Recruitment to and Silencing of P15(INK4b) Tumor Suppressor Gene. Oncogene 2011, 30, 1956–1962. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| RHPN1-AS1-F | GCTCCTGGTCATCAAGTTCCTCT | qRT-PCR |
| RHPN1-AS1-R | GCACAGGCACCAGAATGATCC | qRT-PCR |
| RHPN1-F | TACGACTCGCTTACTGGGGT | qRT-PCR |
| RHPN1-R | GAGGGCACCGATGTTGAAGA | qRT-PCR |
| GAPDH-F | AGGTCGGAGTCAACGGATTTG | qRT-PCR |
| GAPDH-R | TGTAAACCATGTAGTTGAGGTCA | qRT-PCR |
| RHPN1-AS1-si1-sense | CCGAAUCUCUUUACUUCCAdTdT | siRHPN1-AS1 |
| RHPN1-AS1-si1-antisense | UGGAAGUAAAGAGAUUCGGdTdT | siRHPN1-AS1 |
| RHPN1-AS1-si2-sense | CUCAAACUUUGAGGGUCAUdTdT | siRHPN1-AS1 |
| RHPN1-AS1-si2-antisense | AUGACCCUCAAAGUUUGAGdTdT | siRHPN1-AS1 |
| RHPN1-AS1-si3-sense | CUUCCAUACUUCCCUAGGUdTdT | siRHPN1-AS1 |
| RHPN1-AS1-si3-antisense | ACCUAGGGAAGUAUGGAAGdTdT | siRHPN1-AS1 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Yu, X.; Zhang, L.; Ding, X.; Pan, H.; Wen, X.; Xu, S.; Xing, Y.; Fan, J.; Ge, S.; et al. The Long Non-Coding RNA RHPN1-AS1 Promotes Uveal Melanoma Progression. Int. J. Mol. Sci. 2017, 18, 226. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010226
Lu L, Yu X, Zhang L, Ding X, Pan H, Wen X, Xu S, Xing Y, Fan J, Ge S, et al. The Long Non-Coding RNA RHPN1-AS1 Promotes Uveal Melanoma Progression. International Journal of Molecular Sciences. 2017; 18(1):226. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010226
Chicago/Turabian StyleLu, Linna, Xiaoyu Yu, Leilei Zhang, Xia Ding, Hui Pan, Xuyang Wen, Shiqiong Xu, Yue Xing, Jiayan Fan, Shengfang Ge, and et al. 2017. "The Long Non-Coding RNA RHPN1-AS1 Promotes Uveal Melanoma Progression" International Journal of Molecular Sciences 18, no. 1: 226. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010226