Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases
Abstract
:1. Introduction
2. Results
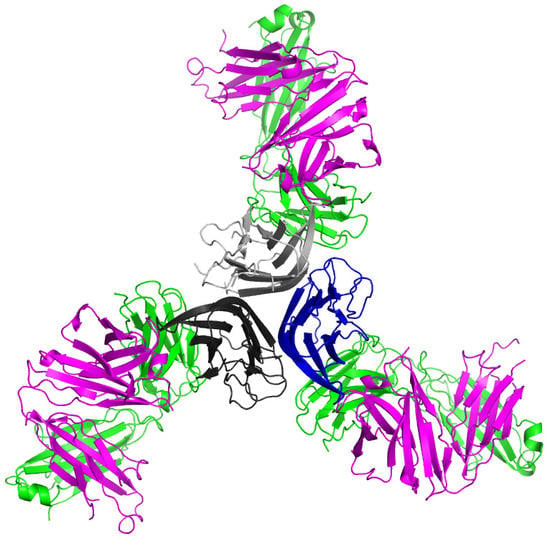
2.1. Crystal Structure of TNFα in Complex with Certolizumab Fab Fragment
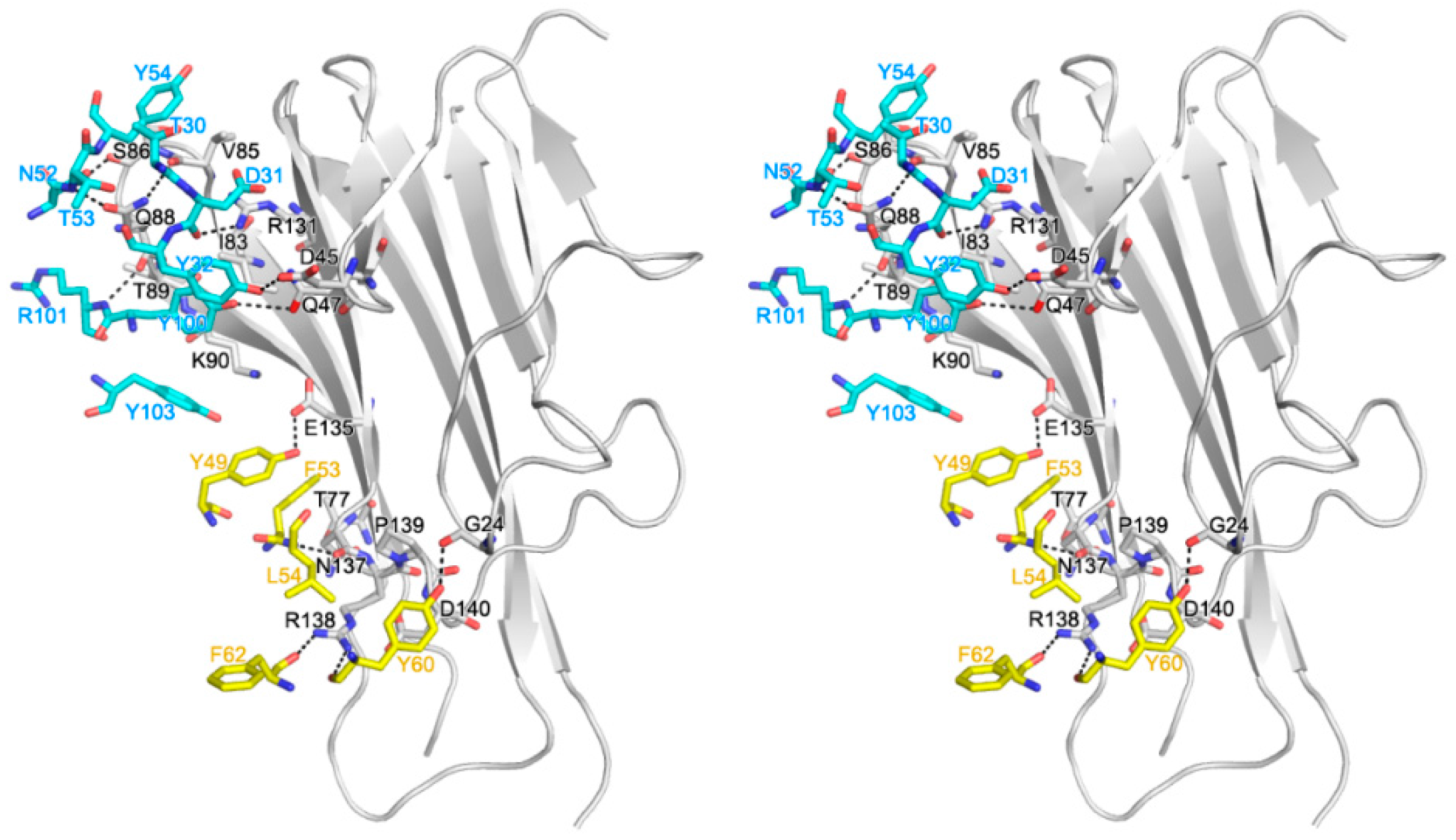
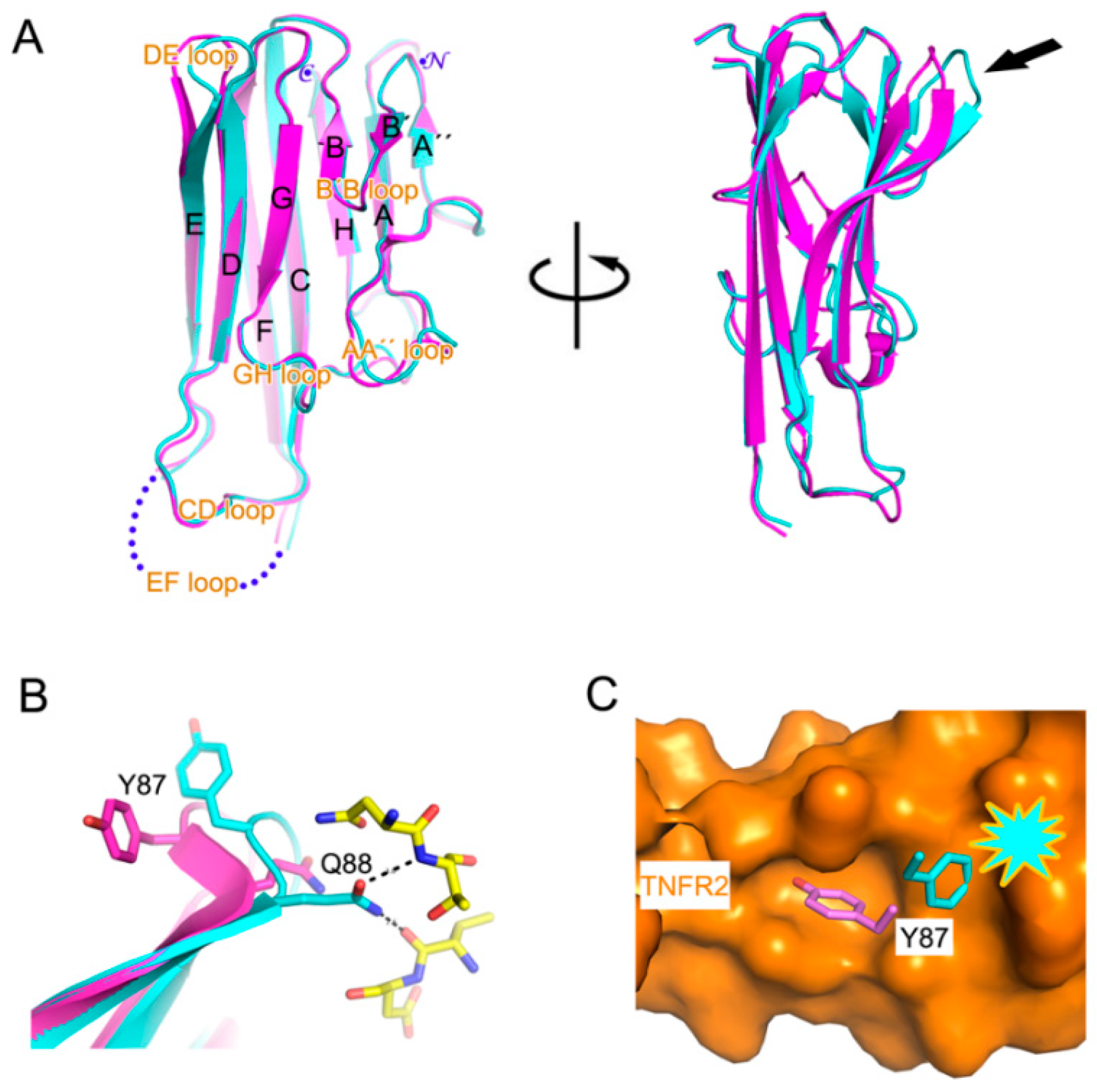
2.2. Interaction between TNF-α and Certolizumab Fab
2.3. Mutagenesis Study of the TNFα-Certolizumab Interface
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of TNFα
4.2. Expression and Purification of the Certolizumab Fab
4.3. Crystallization and Structure Determination of the Certolizumab Fab
4.4. Crystallization and Structure Determination of the TNFα-Certolizumab Fab Complex
4.5. Binding Kinetics of the TNFα WT and Mutants
4.6. Accession Number
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bodmer, J.L.; Schneider, P.; Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 2002, 27, 19–26. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Wiens, G.D.; Glenney, G.W. Origin and evolution of TNF and TNF receptor superfamilies. Dev. Comp. Immunol. 2011, 35, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Bossen, C.; Ingold, K.; Tardivel, A.; Bodmer, J.L.; Gaide, O.; Hertig, S.; Ambrose, C.; Tschopp, J.; Schneider, P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 2006, 281, 13964–13971. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Marušič, J.; Podlipnik, Č.; Jevševar, S.; Kuzman, D.; Vesnaver, G.; Lah, J. Recognition of human tumor necrosis factor α (TNF-α) by therapeutic antibody fragment: Energetics and structural features. J. Biol. Chem. 2012, 287, 8613–8620. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Lam, V.T.; Weber, R.F.; Kohr, W.J.; Basa, L.J.; Spellman, M.W.; Ashkenazi, A.; Shire, S.J.; Goeddel, D.V. Biochemical characterization of the extracellular domain of the 75-kilodalton tumor necrosis factor receptor. Biochemistry 1993, 32, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, H.; Gentz, R.; Zulauf, M.; Lustig, A.; Tabuchi, H.; Schlaeger, E.J.; Brockhaus, M.; Gallati, H.; Manneberg, M.; Lesslauer, W. Recombinant 55-kDa tumor necrosis factor (TNF) receptor. Stoichiometry of binding to TNFα and TNFβ and inhibition of TNF activity. J. Biol. Chem. 1991, 266, 18324–18329. [Google Scholar] [PubMed]
- Eck, M.J.; Sprang, S.R. The structure of tumor necrosis factor-α at 2.6 Å resolution. Implications for receptor binding. J. Biol. Chem. 1989, 264, 17595–17605. [Google Scholar] [PubMed]
- Elliott, M.J.; Maini, R.N.; Feldmann, M.; Kalden, J.R.; Antoni, C.; Smolen, J.S.; Leeb, B.; Breedveld, F.C.; Macfarlane, J.D.; Bijl, H.; et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 1994, 344, 1105–1110. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: The CLASSIC-I trial. Gastroenterology 2006, 130, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Colombo, B.M.; Cagnati, P.; Gulli, R.; Spanò, F.; Puppo, F. Update upon efficacy and safety of TNF-α inhibitors. Expert Opin. Drug Saf. 2012, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Keystone, E.C.; Furst, D.E.; Moreland, L.W.; Weisman, M.H.; Birbara, C.A.; Teoh, L.A.; Fischkoff, S.A.; Chartash, E.K. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheum. 2003, 48, 35–45. [Google Scholar] [CrossRef] [PubMed]
- De Simone, C.; Amerio, P.; Amoruso, G.; Bardazzi, F.; Campanati, A.; Conti, A.; Gisondi, P.; Gualdi, G.; Guarneri, C.; Leoni, L.; et al. Immunogenicity of anti-TNFα therapy in psoriasis: A clinical issue? Expert Opin. Biol. Ther. 2013, 13, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, E.; Weinberg, J.M. Etanercept. Expert Opin. Biol. Ther. 2008, 8, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr. Opin. Pharmacol. 2010, 10, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.D.; Keystone, E.C. Intravenous golimumab in rheumatoid arthritis. Expert Rev. Clin. Immunol. 2014, 10, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Certolizumab Pegol: A Review in inflammatory autoimmune diseases. BioDrugs 2016, 30, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kaymakcalan, Z.; Sakorafas, P.; Bose, S.; Scesney, S.; Xiong, L.; Hanzatian, D.K.; Salfeld, J.; Sasso, E.H. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin. Immunol. 2009, 131, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lis, K.; Kuzawińska, O.; Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors—State of knowledge. Arch. Med. Sci. 2014, 10, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, A. Certolizumab pegol for the management of Crohn’s disease in adults. Clin. Ther. 2009, 31, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Bourne, T.; Fossati, G.; Nesbitt, A. A PEGylated Fab’ fragment against tumor necrosis factor for the treatment of Crohn disease: Exploring a new mechanism of action. BioDrugs 2008, 22, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ishii-Watabe, A.; Tada, M.; Kobayashi, T.; Kanayasu-Toyoda, T.; Kawanishi, T.; Yamaguchi, T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010, 184, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Junghans, R.P.; Anderson, C.L. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G. Pegylation of biological molecules and potential benefits: Pharmacological properties of certolizumab pegol. BioDrugs Suppl. 2014, 1, S15–S23. [Google Scholar] [CrossRef] [PubMed]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Wolf, D.C.; Dubinsky, M.; Cortot, A.; Lee, S.D.; Siegel, C.A.; Ullman, T.; Glover, S.; Valentine, J.F.; Rubin, D.T.; et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.; Airey, M.; Moore, A.; Vugler, A.; Nesbitt, A. Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. J. Immunol. Methods 2009, 348, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, A.; Fossati, G.; Bergin, M.; Stephens, P.; Stephens, S.; Foulkes, R.; Brown, D.; Robinson, M.; Bourne, T. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm. Bowel Dis. 2007, 13, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Tsukamoto, H.; Mitoma, H.; Ayano, M.; Tanaka, A.; Ohta, S.; Inoue, Y.; Arinobu, Y.; Niiro, H.; Akashi, K.; et al. The cytotoxic effects of certolizumab pegol and golimumab mediated by transmembrane tumor necrosis factor α. Inflamm. Bowel Dis. 2013, 19, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Banner, D.W.; D’Arcy, A.; Janes, W.; Gentz, R.; Schoenfeld, H.J.; Broger, C.; Loetscher, H.; Lesslauer, W. Crystal structure of the soluble human 55 kD TNF receptor-human TNFβ complex: Implications for TNF receptor activation. Cell 1993, 73, 431–445. [Google Scholar] [CrossRef]
- Mukai, Y.; Nakamura, T.; Yoshikawa, M.; Yoshioka, Y.; Tsunoda, S.; Nakagawa, S.; Yamagata, Y.; Tsutsumi, Y. Solution of the structure of the TNF–TNFR2 complex. Sci. Signal 2010, 3, ra83. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Dai, J.; Hou, S.; Su, L.; Zhang, D.; Guo, H.; Hu, S.; Wang, H.; Rao, Z.; Guo, Y.; et al. Structural basis for treating tumor necrosis factor α (TNFα)-associated diseases with the therapeutic antibody infliximab. J. Biol. Chem. 2013, 288, 13799–13807. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liang, S.; Guo, H.; Zhang, D.; Li, H.; Wang, X.; Yang, W.; Qian, W.; Hou, S.; Wang, H.; et al. Comparison of the inhibition mechanisms of adalimumab and infliximab in treating tumor necrosis factor α-associated diseases from a molecular view. J. Biol. Chem. 2013, 288, 27059–27067. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Thornton, J.M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, K.A.; Ooijevaar-de Heer, P.; Dijk, L.; Kruithof, S.; Wolbink, G.; Rispens, T. Therapeutic TNF inhibitors can differentially stabilize trimeric TNF by inhibiting monomer exchange. Sci. Rep. 2016, 6, 32747. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.A.; Stanfield, R.L. Antibody-antigen interactions: New structures and new conformational changes. Curr. Opin. Struct. Biol. 1994, 4, 857–867. [Google Scholar] [CrossRef]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular mechanisms of action of anti-TNF-α agents—Comparison among therapeutic TNF-α antagonists. Cytokine 2016. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C. Accessing the Kabat antibody sequence database by computer. Proteins 1996, 25, 130–133. [Google Scholar] [CrossRef]
- Narhi, L.O.; Arakawa, T. Dissociation of recombinant tumor necrosis factor-α studied by gel permeation chromatography. Biochem. Biophys. Res. Commun. 1987, 147, 740–746. [Google Scholar] [CrossRef]
- Corti, A.; Fassina, G.; Marcucci, F.; Barbanti, E.; Cassani, G. Oligomeric tumour necrosis factor α slowly converts into inactive forms at bioactive levels. Biochem. J. 1992, 284, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Hlodan, R.; Pain, R.H. The folding and assembly pathway of tumour necrosis factor TNFα, a globular trimeric protein. Eur. J. Biochem. 1995, 231, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, H.T.; Shin, W.; Chae, J.; Choi, J.; Kim, S.H.; Lim, H.; Heo, T.W.; Park, K.Y.; Lee, Y.J.; et al. Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat. Commun. 2016, 7, 13354. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System; Schrödinger, LLC: New York, NY, USA, 2002. [Google Scholar]




| TNFα | Kon (M−1·s−1) | Koff (s−1) | KD (M) |
|---|---|---|---|
| WT | 1.97 × 106 | 5.40 × 10−5 | 2.74 × 10−11 |
| D45A | 5.86 × 105 | 7.64 × 10−5 | 1.30 × 10−10 |
| Q47A | 5.83 × 105 | 4.39 × 10−5 | 7.53 × 10−11 |
| Q88A | 4.76 × 105 | 2.31 × 10−4 | 4.85 × 10−10 |
| R131A | 3.74 × 105 | 7.42 × 10−5 | 1.98 × 10−10 |
| E135A | 4.60 × 105 | 1.40 × 10−4 | 3.04 × 10−10 |
| R138A | 4.52 × 105 | 2.41 × 10−4 | 5.32 × 10−10 |
| Drug | Trade Name | Type | Approval Date | |
|---|---|---|---|---|
| FDA | EMA | |||
| Etanercept | Enbrel | TNFR2 extracellular portion Fc fusion | 1998 | 2000 |
| Infliximab | Remicade | Chimeric murine/human IgG1 | 1998 | 1999 |
| Adalimumab | Humira | Fully Human IgG1 | 2005 | 2003 |
| Certolizumab-pegol | Cimzia | Humanized, PEGylated Fab’ | 2008 | 2009 |
| Golimumab | Simponi | Fully Human IgG1 | 2009 | 2009 |
| Certolizumab Fab | TNFα-Certolizumab Fab | |
|---|---|---|
| Data Collection | ||
| X-ray source | PLS 5C | PLS 7A |
| Wavelength (Å) | 1.0000 | 1.0000 |
| Space group | P212121 | C2 |
| Cell dimensions | ||
| a, b, c (Å) | 58.33, 63.70, 161.41 | 148.59, 207.22, 112.63 |
| α, β, γ (°) | 90, 90, 90 | 90, 118.81, 90 |
| Resolution (Å) | 1.95 (1.98–1.95) * | 2.89 (2.95–2.89) |
| Rsym (%) | 7.8 (29.7) | 8.1 (48.6) |
| I/σI | 58.1 (3.1) | 19.6.1 (2.3) |
| Completeness (%) | 98.2 (85.6) | 95.2 (94.5) |
| Redundancy | 5.9 (2.5) | 2.9 (2.6) |
| Refinement | ||
| Resolution (Å) | 1.95 | 2.89 |
| No. reflections | 43749 | 63355 |
| Rwork/Rfree (%) | 14.7/17.9 | 22.5/26.5 |
| No. atoms | ||
| Protein | 3290 | 12793 |
| Water | 607 | 0 |
| R.m.s. deviation | ||
| Bond lengths (Å) | 0.007 | 0.006 |
| Bond angles (˚) | 1.060 | 1.278 |
| Ramachandran | ||
| Favored (%) | 98.37 | 95.04 |
| Allowed (%) | 1.63 | 4.47 |
| Outlier (%) | 0.00 | 0.49 |
| PDB code | 5WUV | 5WUX |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.U.; Shin, W.; Son, J.Y.; Yoo, K.-Y.; Heo, Y.-S. Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases. Int. J. Mol. Sci. 2017, 18, 228. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010228
Lee JU, Shin W, Son JY, Yoo K-Y, Heo Y-S. Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases. International Journal of Molecular Sciences. 2017; 18(1):228. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010228
Chicago/Turabian StyleLee, Jee Un, Woori Shin, Ji Young Son, Ki-Young Yoo, and Yong-Seok Heo. 2017. "Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases" International Journal of Molecular Sciences 18, no. 1: 228. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010228