Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities
Abstract
:1. Introduction
2. Stem Cell Sources
2.1. Embryonic Stem Cells (ESCs)
2.2. Neural Stem Cells (NSCs)
2.2.1. Mesenchymal Stem Cells (MSCs)
Bone Marrow-Derived Stem Cells (BMSCs)
Adipose-Derived Stem Cells (ADSCs)
2.2.2. Fetal-Derived Stem Cells
Amniotic Tissue-Derived Stem Cells (ATDSCs)
Umbilical Cord-Derived MSCs (UC-MSCs)
Wharton’s Jelly MSCs (WJMSCs)
2.2.3. Skin-Derived Precursor Stem Cells (SKP-SCs)
2.2.4. Hair Follicle Stem Cells (HFSCs)
2.2.5. Dental Pulp Stem Cells (DPSCs)
2.2.6. Muscle-Derived Stem/Progenitor Cells (MDSPCs)
2.3. Induced Pluripotential Stem Cells (iPSCs)
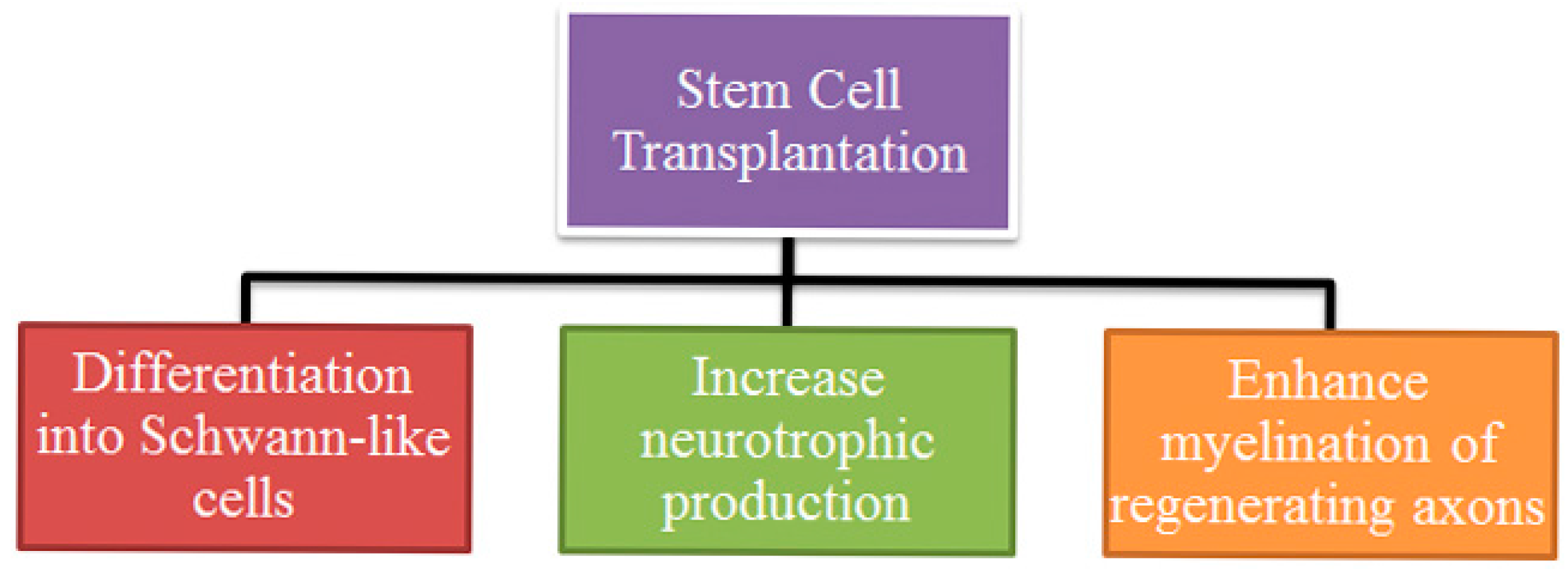
3. Mechanism of Action
3.1. Differentiation Type of Stem Cells
3.2. Neurotrophic Action Enhancement
3.3. Myelin Promotion
4. Stem Cell Delivery
5. Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Bronckaers, A.; Politis, C.; Jacobs, R.; Lambrichts, I. Dental stem cells and their promising role in neural regeneration: An update. Clin. Oral Investig. 2013, 17, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippon, H.J.; Bishop, A.E. Embryonic stem cells. Cell Prolif. 2004, 37, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, L.; Grigoryan, S.; Yang, I.H.; Thakor, N.V.; Goldstein, R.S. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev. Rep. 2011, 7, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jiang, J.; Wei, L.; Zhou, X.; Fraser, J.L.; Snider, B.J.; Yu, S.P. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells 2008, 26, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Randolph, M.A.; Groger, A.; Winograd, J.M. Embryonic stem cell-derived motor neurons form neuromuscular junctions in vitro and enhance motor functional recovery in vivo. Plast. Reconstr. Surg. 2009, 123, 139S–148S. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Lye, E.; Suan Yeo, K.; Khia Way Tan, E.; Salto-Tellez, M.; Liu, T.M.; Palanisamy, N.; El Oakley, R.M.; Lee, E.H.; Lim, B.; et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells 2007, 25, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Xu, L.; Kim, G.H.; Kang, S.K.; Lee, S.W.; Park, S.H.; Kim, S.; Choi, T.H.; Kim, H.S. Regeneration of peripheral nerves by transplanted sphere of human mesenchymal stem cells derived from embryonic stem cells. Biomaterials 2012, 33, 7039–7046. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Tetzlaff, W.; Weiss, S. A multipotent egf-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992, 12, 4565–4574. [Google Scholar] [PubMed]
- Snyder, E.Y.; Deitcher, D.L.; Walsh, C.; Arnold-Aldea, S.; Hartwieg, E.A.; Cepko, C.L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 1992, 68, 33–51. [Google Scholar] [CrossRef]
- Paspala, S.A.; Murthy, T.V.; Mahaboob, V.S.; Habeeb, M.A. Pluripotent stem cells—A review of the current status in neural regeneration. Neurol. India 2011, 59, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.T.; Schafer, S.T.; Gage, F.H. Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Benton, R.L.; Whittemore, S.R. Stem cell repair of central nervous system injury. J. Neurosci. Res. 2002, 68, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Heine, W.; Conant, K.; Griffin, J.W.; Hoke, A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp. Neurol. 2004, 189, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Chen, J.H.; Hsu, T.Y.; Chang, L.H.; Chang, H.; Chi, Y.H.; Chiu, I.M. Neural stem cells promote nerve regeneration through IL12-induced schwann cell differentiation. Mol. Cell. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.S.; O’Neill, A.C.; Motarjem, P.M.; Nazzal, J.; Randolph, M.; Winograd, J.M. Tumor formation following murine neural precursor cell transplantation in a rat peripheral nerve injury model. J. Reconstr. Microsurg. 2008, 24, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisen, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, Y.T.; Tsang, K.S.; Sun, C.R.; Li, J.; Huang, H.; Cui, F.Z.; An, Y.H. Implantation of neural stem cells embedded in hyaluronic acid and collagen composite conduit promotes regeneration in a rabbit facial nerve injury model. J. Transl. Med. 2008, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Maltman, D.J.; Hardy, S.A.; Przyborski, S.A. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem. Int. 2011, 59, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Tohill, M.; Terenghi, G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol. Appl. Biochem. 2004, 40, 17–24. [Google Scholar] [PubMed]
- Cuevas, P.; Carceller, F.; Dujovny, M.; Garcia-Gomez, I.; Cuevas, B.; Gonzalez-Corrochano, R.; Diaz-Gonzalez, D.; Reimers, D. Peripheral nerve regeneration by bone marrow stromal cells. Neurol. Res. 2002, 24, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, T.H.; Bodar, C.W.; van Neck, J.W.; Walbeehm, E.T.; Siemionow, M.; Madajka, M.; Cwykiel, J.; Blok, J.H.; Hovius, S.E. Natural conduits for bridging a 15-mm nerve defect: Comparison of the vein supported by muscle and bone marrow stromal cells with a nerve autograft. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2013, 66, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.W.; Luo, M.; Li, Y.J.; Zhang, K.Q. Biological conduits combining bone marrow mesenchymal stem cells and extracellular matrix to treat long-segment sciatic nerve defects. Neural Regen. Res. 2015, 10, 965–971. [Google Scholar] [PubMed]
- Carrier-Ruiz, A.; Evaristo-Mendonca, F.; Mendez-Otero, R.; Ribeiro-Resende, V.T. Biological behavior of mesenchymal stem cells on poly-epsilon-caprolactone filaments and a strategy for tissue engineering of segments of the peripheral nerves. Stem Cell Res. Ther. 2015, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Raheja, A.; Suri, V.; Suri, A.; Sarkar, C.; Srivastava, A.; Mohanty, S.; Jain, K.G.; Sharma, M.C.; Mallick, H.N.; Yadav, P.K.; et al. Dose-dependent facilitation of peripheral nerve regeneration by bone marrow-derived mononuclear cells: A randomized controlled study: Laboratory investigation. J. Neurosurg. 2012, 117, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, X.; Yang, Q.; Wang, L.; Sun, J.; Zhan, H.; Lin, J.; Pu, Z.; Jiang, J.; Sun, Y.; et al. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: The China-AMI randomized controlled trial. Int. J. Cardiol. 2015, 184, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, S.F.; van Ramshorst, J.; Mann, I.; Leong, D.P.; Cannegieter, S.C.; Al Younis, I.; Dibbets-Schneider, P.; de Roos, A.; Fibbe, W.E.; Zwaginga, J.J.; et al. Predictors of response to intramyocardial bone marrow cell treatment in patients with refractory angina and chronic myocardial ischemia. Int. J. Cardiol. 2014, 175, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Kumar, S.R.; Narayanan, R.; Arul, K.; Baskaran, M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp. Clin. Transplant. 2009, 7, 241–248. [Google Scholar] [PubMed]
- Gimble, J.M.; Bunnell, B.A.; Frazier, T.; Rowan, B.; Shah, F.; Thomas-Porch, C.; Wu, X. Adipose-derived stromal/stem cells: A primer. Organogenesis 2013, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Lin, G.; Lue, T.F.; Lin, C.S. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 2006, 74, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, C.; Raimondo, S.; Haneef, M.S.; Geuna, S.; Terenghi, G.; Shawcross, S.G.; Wiberg, M. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp. Cell Res. 2012, 318, 2034–2048. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.H.; Novikov, L.N.; Wiberg, M.; Kingham, P.J. Intrinsic mechanisms underlying the neurotrophic activity of adipose derived stem cells. Exp. Cell Res. 2015, 331, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Sowa, Y.; Kishida, T.; Imura, T.; Numajiri, T.; Nishino, K.; Tabata, Y.; Mazda, O. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into schwann-like lineage. Plast. Reconstr. Surg. 2016, 137, 318e–330e. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, S.; Tada, K.; Hayashi, K.; Takeuchi, A.; Sugimoto, N.; Ikeda, K.; Tsuchiya, H. Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration. J. Orthop. Sci. 2013, 18, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.M.; Vykoukal, J.; Li, D.P.; Pan, H.L.; Zeitler, K.; Alt, E.; Geis, S.; Felthaus, O.; Prantl, L. Peripheral motor and sensory nerve conduction following transplantation of undifferentiated autologous adipose tissue-derived stem cells in a biodegradable US Food and drug administration-approved nerve conduit. Plast. Reconstr. Surg. 2016, 138, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.B.; Singh, P.; Feaster, M.M.; Ramnarain, A.; Pavlides, C.; Chen, Z.L.; Yu, W.M.; Feltri, M.L.; Strickland, S. Mesenchymal stem cells facilitate axon sorting, myelination, and functional recovery in paralyzed mice deficient in schwann cell-derived laminin. Glia 2011, 59, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Lasso, J.M.; Perez Cano, R.; Castro, Y.; Arenas, L.; Garcia, J.; Fernandez-Santos, M.E. Xenotransplantation of human adipose-derived stem cells in the regeneration of a rabbit peripheral nerve. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2015, 68, e189–e197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wei, X.; Ma, Z.; Feng, D.; Tu, P.; Johnstone, B.H.; March, K.L.; Du, Y. Adipose stromal cells-conditional medium protected glutamate-induced cgns neuronal death by bdnf. Neurosci. Lett. 2009, 452, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Sowa, Y.; Imura, T.; Numajiri, T.; Nishino, K.; Fushiki, S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: Influence of age and anatomic site of origin. Stem Cells Dev. 2012, 21, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.; Mantovani, C.; Kalbermatten, D.F.; Pierer, G.; Terenghi, G.; Kingham, P.J. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2010, 63, e811–e817. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Yang, D.; Xu, J.; Si, Z.; Jin, X.; Zhang, J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011, 20, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Sun, C.K.; Lin, Y.C.; Chang, L.T.; Chen, Y.L.; Tsai, T.H.; Chung, S.Y.; Chua, S.; Kao, Y.H.; Yen, C.H.; et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J. Transl. Med. 2011, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, P.E.; Tremp, M.; Kingham, P.J.; di Summa, P.G.; Largo, R.D.; Schaefer, D.J.; Kalbermatten, D.F. Harvest site influences the growth properties of adipose derived stem cells. Cytotechnology 2013, 65, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Tremp, M.; Meyer Zu Schwabedissen, M.; Kappos, E.A.; Engels, P.E.; Fischmann, A.; Scherberich, A.; Schaefer, D.J.; Kalbermatten, D.F. The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transplant. 2015, 24, 203–211. [Google Scholar] [PubMed]
- Faroni, A.; Smith, R.J.; Lu, L.; Reid, A.J. Human schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2016, 43, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, N.G.; Randolph, M.A.; Redmond, R.W. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2014, 67, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Cheng, Y.C.; Lin, M.Y.; Cheng, H.; Chu, P.M.; Chou, S.C.; Shih, Y.H.; Ko, M.H.; Sung, M.S. Conversion of human umbilical cord mesenchymal stem cells in wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for parkinsonism. Stem Cells 2006, 24, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Hwang, S.M.; Tsai, Y.L.; Cheng, F.C.; Lee, J.L.; Chang, Y.J. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol. Reprod. 2006, 74, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lee, J.L.; Chang, Y.J.; Hwang, S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004, 19, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, L.; Ahn, H.S.; Kim, M.H.; Kim, S.W. Amniotic mesenchymal stem cells display neurovascular tropism and aid in the recovery of injured peripheral nerves. J. Cell. Mol. Med. 2014, 18, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Cheng, F.C.; Chen, C.J.; Lai, S.Z.; Lee, C.W.; Yang, D.Y.; Chang, M.H.; Ho, S.P. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J. Clin. Neurosci. 2007, 14, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Yang, D.Y.; Ho, S.P.; Sheu, M.L.; Chen, C.J.; Hwang, S.M.; Chang, M.H.; Cheng, F.C. Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, natto. J. Biomed. Sci. 2009, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.C.; Tai, M.H.; Sheu, M.L.; Chen, C.J.; Yang, D.Y.; Su, H.L.; Ho, S.P.; Lai, S.Z.; Pan, H.C. Enhancement of regeneration with glia cell line-derived neurotrophic factor-transduced human amniotic fluid mesenchymal stem cells after sciatic nerve crush injury. J. Neurosurg. 2010, 112, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Y.; Sheu, M.L.; Su, H.L.; Cheng, F.C.; Chen, Y.J.; Chen, C.J.; Chiu, W.T.; Yiin, J.J.; Sheehan, J.; Pan, H.C. Dual regeneration of muscle and nerve by intravenous administration of human amniotic fluid-derived mesenchymal stem cells regulated by stromal cell-derived factor-1alpha in a sciatic nerve injury model. J. Neurosurg. 2012, 116, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.Y.; Gauthaman, K. Taking stem cells to the clinic: Major challenges. J. Cell. Biochem. 2008, 105, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Sun, X.; Xu, X.L.; Zhao, Q.; Peng, J.; Wang, Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen. Res. 2015, 10, 651–658. [Google Scholar] [PubMed]
- Zarbakhsh, S.; Goudarzi, N.; Shirmohammadi, M.; Safari, M. Histological study of bone marrow and umbilical cord stromal cell transplantation in regenerating rat peripheral nerve. Cell J. 2016, 17, 668–677. [Google Scholar] [PubMed]
- Raio, L.; Ghezzi, F.; Di Naro, E.; Gomez, R.; Franchi, M.; Mazor, M.; Bruhwiler, H. Sonographic measurement of the umbilical cord and fetal anthropometric parameters. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 83, 131–135. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.L.; Zhu, J.K.; Jiang, L.; Hu, J.; Zhang, Y.; Yang, L.M.; Wang, H.G.; Yi, J.H. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008, 1188, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, Y.; Zhang, L.; Zhao, B.; Zhao, Z.; Chen, J.; Guo, Q.; Liu, S.; Sui, X.; Xu, W.; et al. Human umbilical cord wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res. Bull. 2011, 84, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.A.; McKenzie, I.A.; Toma, J.G.; Miller, F.D. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat. Protoc. 2006, 1, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef] [PubMed]
- Khuong, H.T.; Kumar, R.; Senjaya, F.; Grochmal, J.; Ivanovic, A.; Shakhbazau, A.; Forden, J.; Webb, A.; Biernaskie, J.; Midha, R. Skin derived precursor schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 2014, 254, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Grimoldi, N.; Colleoni, F.; Tiberio, F.; Vetrano, I.G.; Cappellari, A.; Costa, A.; Belicchi, M.; Razini, P.; Giordano, R.; Spagnoli, D.; et al. Stem cell salvage of injured peripheral nerve. Cell Transplant. 2015, 24, 213–222. [Google Scholar] [PubMed]
- Joannides, A.; Gaughwin, P.; Schwiening, C.; Majed, H.; Sterling, J.; Compston, A.; Chandran, S. Efficient generation of neural precursors from adult human skin: Astrocytes promote neurogenesis from skin-derived stem cells. Lancet 2004, 364, 172–178. [Google Scholar] [CrossRef]
- Yu, H.; Kumar, S.M.; Kossenkov, A.V.; Showe, L.; Xu, X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J. Investig. Dermatol. 2010, 130, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Amoh, Y.; Aki, R.; Hamada, Y.; Niiyama, S.; Eshima, K.; Kawahara, K.; Sato, Y.; Tani, Y.; Hoffman, R.M.; Katsuoka, K. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J. Dermatol. 2012, 39, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, F.; Zhang, C.; Zhang, Z.; Guo, J.; Ren, C.; Kong, Z. Pluripotent hair follicle neural crest stem-cell-derived neurons and schwann cells functionally repair sciatic nerves in rats. Mol. Neurobiol. 2009, 40, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Yaghoobi, M.M.; Shamsara, M.; Esmaeili-Mahani, S. Tetracycline-regulated expression of OLIG2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. Neuroscience 2015, 305, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Shi, S.; Zannettino, A.C.; Fujii, N.; Gronthos, S.; Koblar, S.A. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells 2009, 27, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Osako, Y.; Ito, M.; Murakami, M.; Hayashi, Y.; Horibe, H.; Iohara, K.; Takeuchi, N.; Okui, N.; Hirata, H.; et al. Trophic effects of dental pulp stem cells on Schwann cells in peripheral nerve regeneration. Cell Transplant. 2016, 25, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sugimura-Wakayama, Y.; Katagiri, W.; Osugi, M.; Kawai, T.; Ogata, K.; Sakaguchi, K.; Hibi, H. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev. 2015, 24, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.M.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hei, W.H.; Kim, S.; Park, J.C.; Seo, Y.K.; Kim, S.M.; Jahng, J.W.; Lee, J.H. Schwann-like cells differentiated from human dental pulp stem cells combined with a pulsed electromagnetic field can improve peripheral nerve regeneration. Bioelectromagnetics 2016, 37, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Deasy, B.M.; Gharaibeh, B.M.; Pollett, J.B.; Jones, M.M.; Lucas, M.A.; Kanda, Y.; Huard, J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol. Biol. Cell 2005, 16, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A.; et al. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J. Cell Biol. 2002, 157, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, M.; Thompson, S.D.; Pollett, J.B.; Usas, A.; Lu, A.; Stolz, D.B.; Clark, K.A.; Sun, B.; Peault, B.; Huard, J. Human muscle-derived stem/progenitor cells promote functional murine peripheral nerve regeneration. J. Clin. Investig. 2014, 124, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Soeda, S.; Hashimoto, H.; Saito, K.; Sakai, A.; Nakajima, N.; Masuda, M.; Fukunishi, N.; Uchiyama, Y.; Terachi, T.; et al. 3d reconstitution of nerve-blood vessel networks using skeletal muscle-derived multipotent stem cell sheet pellets. Regen. Med. 2013, 8, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Bulken-Hoover, J.D.; Jackson, W.M.; Ji, Y.; Volger, J.A.; Tuan, R.S.; Nesti, L.J. Inducible expression of neurotrophic factors by mesenchymal progenitor cells derived from traumatically injured human muscle. Mol. Biotechnol. 2012, 51, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Robinton, D.A.; Daley, G.Q. The promise of induced pluripotent stem cells in research and therapy. Nature 2012, 481, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Denham, M.; Dottori, M. Neural differentiation of induced pluripotent stem cells. Methods Mol. Biol. 2011, 793, 99–110. [Google Scholar] [PubMed]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Uemura, T.; Takamatsu, K.; Okada, M.; Kazuki, K.; Tabata, Y.; Ikada, Y.; Nakamura, H. Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J. Biomed. Mater. Res. Part A 2014, 102, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Takamatsu, K.; Ikeda, M.; Okada, M.; Kazuki, K.; Ikada, Y.; Nakamura, H. A tissue-engineered bioabsorbable nerve conduit created by three-dimensional culture of induced pluripotent stem cell-derived neurospheres. Bio-Med. Mater. Eng. 2011, 21, 333–339. [Google Scholar]
- Uemura, T.; Ikeda, M.; Takamatsu, K.; Yokoi, T.; Okada, M.; Nakamura, H. Long-term efficacy and safety outcomes of transplantation of induced pluripotent stem cell-derived neurospheres with bioabsorbable nerve conduits for peripheral nerve regeneration in mice. Cells Tissues Organs 2014, 200, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.J.; DeWitt, J.C.; Setty, N.; Donald, M.D.; Joo, E.; Chesarone, M.A.; Birren, S.J. Multiple signaling pathways converge to regulate bone-morphogenetic-protein-dependent glial gene expression. Dev. Neurosci. 2009, 31, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Kanno, H.; Hoshino, M.; Cho, H.; Matsumoto, N.; Itokazu, Y.; Tajima, N.; Yamada, H.; Sawada, H.; Ishikawa, H.; et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Investig. 2004, 113, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Teng, F.Y.; Tang, B.L. Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: Progress and uncertainties. Cell. Mol. Life Sci. 2006, 63, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.S.; Boddeke, E.; Copray, S. Pluripotent stem cells for schwann cell engineering. Stem Cell Rev. 2015, 11, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Orbay, H.; Uysal, A.C.; Hyakusoku, H.; Mizuno, H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Shea, G.K.; Tsui, A.Y.; Chan, Y.S.; Shum, D.K. Bone marrow-derived schwann cells achieve fate commitment—A prerequisite for remyelination therapy. Exp. Neurol. 2010, 224, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, E.; Li, Y.; Hu, J. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 2011, 1383, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Madura, T.; Mantovani, C.; Terenghi, G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J. Neurosci. Res. 2012, 90, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Di Summa, P.G.; Kingham, P.J.; Campisi, C.C.; Raffoul, W.; Kalbermatten, D.F. Collagen (NeuraGen®) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci. Lett. 2014, 572, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Swijnenburg, R.J.; Schrepfer, S.; Cao, F.; Pearl, J.I.; Xie, X.; Connolly, A.J.; Robbins, R.C.; Wu, J.C. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008, 17, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Midha, R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg. Focus 2009, 26, E2. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Long, Y.; Yuan, X.; Zou, L.; Sun, J.; Chen, S.; Perez-Polo, J.R.; Yang, K. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: Synthesis of neurotrophic factors. J. Neurosci. Res. 2005, 80, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Sun, M.; Wiberg, M.; Downes, S.; Terenghi, G.; Kingham, P.J. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience 2011, 199, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Resende, V.T.; Pimentel-Coelho, P.M.; Mesentier-Louro, L.A.; Mendez, R.M.; Mello-Silva, J.P.; Cabral-da-Silva, M.C.; de Mello, F.G.; de Melo Reis, R.A.; Mendez-Otero, R. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience 2009, 159, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Pavlova, G.; Parfyonova, Y.; Tkachuk, V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: Bdnf secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE 2011, 6, e17899. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, L.; Li, Y.; Zhou, C.; Xiong, F.; Liu, Z.; Gu, R.; Hou, X.; Zhang, C. Myelin-forming ability of schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 2008, 1239, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Garbay, B.; Heape, A.M.; Sargueil, F.; Cassagne, C. Myelin synthesis in the peripheral nervous system. Prog. Neurobiol. 2000, 61, 267–304. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.; Xu, Q.; Park, W.; Lee, S.; Furuhashi, A.; Le, A.D. Neural progenitor-like cells induced from human gingiva-derived mesenchymal stem cells regulate myelination of schwann cells in rat sciatic nerve regeneration. Stem Cells Transl. Med. 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.J.; Tong, L.; Ji, L.L.; Wang, Z.Y.; Zhang, X.; Gao, H.; Jia, H.; Zhang, L.X.; Tong, X.J. Synergistic effects of ultrashort wave and bone marrow stromal cells on nerve regeneration with acellular nerve allografts. Synapse 2013, 67, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Y.; Peng, J.; Ren, Z.; Zhang, L.; Guo, Q.; Xu, W.; Lu, S. Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin. Cell Transplant. 2014, 23, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Whiteley, R.; Yu, T.; Stringer, J.; MacNeil, S.; Haycock, J.W.; Smith, P.J. Inkjet printing Schwann cells and neuronal analogue NG108–15 cells. Biofabrication 2016, 8, 015017. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Jia, X. 3D printed nerve guidance channels: Computer-aided control of geometry, physical cues, biological supplements and gradients. Neural. Regen. Res. 2016, 11, 1568–1569. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3d printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Weightman, A.; Jenkins, S.; Pickard, M.; Chari, D.; Yang, Y. Alignment of multiple glial cell populations in 3D nanofiber scaffolds: Toward the development of multicellular implantable scaffolds for repair of neural injury. Nanomedicine 2014, 10, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Y.; Gou, Z.; Tao, J.; Zhang, J.; Liu, Q.; Kang, T.; Jiang, S.; Huang, S.; He, J.; et al. 3D-engineering of cellularized conduits for peripheral nerve regeneration. Sci. Rep. 2016, 6, 32184. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.J.; Ferreira Bento, R.; Salomone, R.; Azzi-Nogueira, D.; Zanatta, D.B.; Paulino Costa, M.; da Silva, C.F.; Strauss, B.E.; Haddad, L.A. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013, 1510, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, X.; Ding, F.; Yao, D.; Gu, Y.; Liu, J.; Gu, X. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng. Part A 2011, 17, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Hayashi, T.; Kitada, M.; Kohama, M.; Matsue, D.; Teramoto, N.; Ose, T.; Itokazu, Y.; Koshino, K.; Watabe, H.; et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp. Neurol. 2010, 223, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Pereira Lopes, F.R.; Camargo de Moura Campos, L.; Dias Correa J., Jr.; Balduino, A.; Lora, S.; Langone, F.; Borojevic, R.; Blanco Martinez, A.M. Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp. Neurol. 2006, 198, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Di Summa, P.G.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Kingham, P.J.; Terenghi, G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wu, H.; Xue, C.; Gong, Y.; Wu, J.; Xiao, Z.; Yang, Y.; Ding, F.; Gu, X. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials 2013, 34, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Z.; Huang, J.; Wang, H.; Gu, X.; Gu, J. Application of marrow mesenchymal stem cell-derived extracellular matrix in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tohill, M.; Mantovani, C.; Wiberg, M.; Terenghi, G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 2004, 362, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Salomone, R.; Bento, R.F.; Costa, H.J.; Azzi-Nogueira, D.; Ovando, P.C.; Da-Silva, C.F.; Zanatta, D.B.; Strauss, B.E.; Haddad, L.A. Bone marrow stem cells in facial nerve regeneration from isolated stumps. Muscle Nerve 2013, 48, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kappos, E.A.; Engels, P.E.; Tremp, M.; Meyer zu Schwabedissen, M.; di Summa, P.; Fischmann, A.; von Felten, S.; Scherberich, A.; Schaefer, D.J.; Kalbermatten, D.F. Peripheral nerve repair: Multimodal comparison of the long-term regenerative potential of adipose tissue-derived cells in a biodegradable conduit. Stem Cells Dev. 2015, 24, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.H.; Haycock, J.W. Next generation nerve guides: Materials, fabrication, growth factors, and cell delivery. Tissue Eng. Part B Rev. 2012, 18, 116–128. [Google Scholar] [CrossRef] [PubMed]
| Stem Cell | Classification | Advantage | Disadvantage | Preclinical or Clinical Use | Mechanism |
|---|---|---|---|---|---|
| ESCs | Pluripotent stem cells | Homogenous, no detrimental impact of age and disease, unlimited cell number, better differentiation potential, and longer lasting proliferation capacity | Teratoma formation, ethical dilemma | Preclinical [8,9] | Myelination and/or neurotrophic factors |
| NSCs | Multipotent stem cells | Difficult to be harvested | Preclinical [15,16] | Replace Schwann cells | |
| BMSCs | Multipotent cells | Easily accessible without ethical concerns | Lower capacity of proliferation and differentiation, invasive procedure for autologous harvesting | Preclinical [25,26] | Myelination, neurotrophic factors |
| ADSCs | Multipotent stem cells | Easy to harvest, higher proportion and superior proliferation | Differentiation potential towards adipocytes | Preclinical [40,41,42,43] | Myelination, neurotrophic factors, reduce inflammation |
| Fetal-derived stem cell | Multipotent stem cells | Less immunoreactivity | Cell bank for storage | Preclinical [57,58,65,68] | Augmented blood perfusion and enhanced intraneural vascularity |
| SKP-SCs | Multipotent cells | Easy to harvest | Long time to differentiate | Preclinical [71] | Replace Schwann cell myelination |
| HFSCs | Multipotent stem cells | Abundant and accessible source, differentiate into pure human SC population | Difficult to isolate | Preclinical [75] | Replace Schwann cell myelination, neurotrophic factors |
| DPSCs | Multipotent stem cells | Stronger harvesting and proliferation potential, as well as greater clonogenic potential | Require storage | Preclinical [80,81] | Replace Schwann cell myelination, neurotrophic factors |
| MDSPCs | Progenitor cells | Abundant and accessible source | Limited research | Preclinical [89] | Neurotrophic factors |
| iPSCs | Pluripotent stem cells | Inducible from easily obtainable somatic cells | Subdued efficiency and enhanced variability during the differentiation process, epigenetic memory from the original somatic cells, chromosomal aberrations, stronger tumorigenicity | Preclinical [93] | Replace Schwann cell myelination, neurotrophic factors |
| Methods | Application | Advantage and Disadvantage | References |
|---|---|---|---|
| Micro injection | Traumatic both to the stem cells and delicate intra-neural architecture, abnormal cell distribution | Pang [116] | |
| Conduit | Natural conduits or artificial | Difficult for cell delivery | Nijhuis [25] Costa [123] Yang [124] Carrier-Ruiz [27] Wakao [125] |
| Conduit + ECM | Collagen, fibirin | Good cell distribution, lack of 3-D construction | Pereira [126] di Summa [127] |
| Conduit + internal | Beneficial for axonal guidance | Wakao [125] Hu [128] Gu [129] | |
| 3-D print | Customization, good cell distribution | Weightman [121] Hu [122] Tse [118] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Jones, S.; Jia, X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int. J. Mol. Sci. 2017, 18, 94. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010094
Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. International Journal of Molecular Sciences. 2017; 18(1):94. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010094
Chicago/Turabian StyleJiang, Liangfu, Salazar Jones, and Xiaofeng Jia. 2017. "Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities" International Journal of Molecular Sciences 18, no. 1: 94. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010094







