Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources
Abstract
:1. Introduction
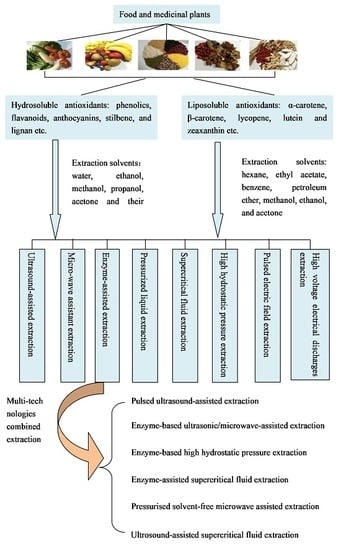
2. Extraction Methods of Antioxidants from Foods and Medicinal Plants
2.1. Ultrasound-Assisted Extraction (UAE)
2.2. Microwave-Assisted Extraction (MAE)
2.3. Enzyme-Assisted Extraction (EAE)
2.4. Pressurized Liquid Extraction (PLE)
2.5. Supercritical Fluid Extraction (SFE)
2.6. Others
3. Assessment Methods of Antioxidant Capacity
3.1. Chemical-Based Assays
3.1.1. Scavenging Free Radicals Assays
3.1.2. Reducing the Metal Ions Assays (FRAP and CUPRAC Assays)
3.1.3. Folin–Ciocalteu Reagent (FCR) Assay
3.1.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.1.5. Total Radical Trapping Antioxidant Potential (TRAP) Assay
3.1.6. Inhibiting the Oxidation of Low-Density Lipoprotein (LDL) Assay
3.2. Cellular-Based Assays
4. Main Resources of Natural Antioxidants
4.1. Natural Sources of Polyphenols
4.2. Natural Sources of Carotenoids
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. BioMed Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Li, Y.; Zhang, Y.J.; Zhou, Y.; Li, S.; Li, H.B. Natural products for the prevention and treatment of hangover and alcohol use disorder. Molecules 2016, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, S.; Zhou, T.; Zhang, P.; Li, H.B. Alcoholic beverage consumption and chronic diseases. Int. J. Environ. Res. Public Health 2016, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; del Nobile, M.A. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 2015, 56, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from south China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Food. 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Food 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Food 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.J.; Xu, D.P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H.B. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Li, Y.; Zhou, T.; Xu, D.P.; Zhang, P.; Li, S.; Li, H.B. Bioactivities and health benefits of mushrooms mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.L.; Lin, X.; Xie, J.F.; Xia, E.Q. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Jenab, M.; Riboli, E.; Ferrari, P.; Sabate, J.; Slimani, N.; Norat, T.; Friesen, M.; Tjonneland, A.; Olsen, A.; Overvad, K.; et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006, 27, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Arathi, B.P.; Raghavendra-Rao Sowmya, P.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of carotenoids: The challenges and prospects—A review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Oniszczuk, T.; Waksmundzka-Hajnos, M. The influence of common free radicals and antioxidants on development of Alzheimer’s disease. Biomed. Pharmacother. 2016, 78, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N. Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer’s disease. Mech. Ageing Dev. 2016, 153, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, A.C.; Bismara Regitano-d’Arce, M.A.; Telles Biasoto, A.C.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of α-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Nikitha, V.S.; Ilaiyarasi, S.; Dhivya, K.; Rajasekar, V.; Kumar, A.N.M.; Muthukumaran, K.; Muthukumaran, C. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2016, 84, 13–21. [Google Scholar] [CrossRef]
- Van Tang, N.; Hong, N.T.P.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Influence of solvents and novel extraction methods on bioactive compounds and antioxidant capacity of Phyllanthus amarus. Chem. Pap. 2016, 70, 556–566. [Google Scholar]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; Martin-Gonzalez, M.F.S. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT-Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Faidi, K.; Baaka, N.; Hammami, S.; El Mokni, R.; Mighri, Z.; Mhenni, M.F. Extraction of carotenoids from Lycium ferocissimum fruits for cotton dyeing: Optimization survey based on a central composite design method. Fibers Polym. 2016, 17, 36–43. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2016, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Xu, D.P.; Xu, X.R.; Chen, Y.M.; Ling, W.H.; Chen, F.; Li, H.B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal. Meth. 2015, 8, 1207–1214. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, M.; Radovanovic, N.; Corovic, M.; Siler-Marinkovic, S.; Rajilic-Stojanovic, M.; Dimitrijevic-Brankovic, S. Optimisation of microwave-assisted extraction parameters for antioxidants from waste Achillea millefolium dust. Ind. Crop. Prod. 2015, 77, 333–341. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crop. Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupic, D.; Preiner, D.; Asperger, D.; Kontic, J.K. Recovery of flavonoids from grape skins by enzyme-assisted extraction. Sep. Sci. Technol. 2016, 51, 255–268. [Google Scholar] [CrossRef]
- Ranveer, R.C.; Patil, S.N.; Sahoo, A.K. Effect of different parameters on enzyme-assisted extraction of lycopene from tomato processing waste. Food Bioprod. Process. 2013, 91, 370–375. [Google Scholar] [CrossRef]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F.; Ghaziaskar, S.H. Optimization of phenolic and flavonoid content and antioxidants capacity of pressurized liquid extraction from Dracocephalum kotschyi via circumscribed central composite. J. Supercrit. Fluids 2016, 107, 307–314. [Google Scholar] [CrossRef]
- Golmakani, E.; Mohammadi, A.; Sani, T.A.; Kamali, H. Phenolic and flavonoid content and antioxidants capacity of pressurized liquid extraction and perculation method from roots of Scutellaria pinnatifida a. Hamilt. Subsp alpina (Bornm) Rech. F. J. Supercrit. Fluids 2014, 95, 318–324. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Um, B. Optimisation of pressurised liquid extraction of antioxidants from black bamboo leaves. Food Chem. 2014, 154, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sanagi, M.M.; See, H.H.; Ibrahim, W.; Abu Naim, A. Determination of carotene, tocopherols and tocotrienols in residue oil from palm pressed fiber using pressurized liquid extraction-normal phase liquid chromatography. Anal. Chim. Acta 2005, 538, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.; Cebola, M.J.; Oliveira, M.C.; Bernardo-Gil, M.G. Supercritical fluid extraction vs. conventional extraction of myrtle leaves and berries: Comparison of antioxidant activity and identification of bioactive compounds. J. Supercrit. Fluids 2016, 113, 1–9. [Google Scholar] [CrossRef]
- Kazan, A.; Koyu, H.; Turu, I.C.; Yesil-Celiktas, O. Supercritical fluid extraction of Prunus persica leaves and utilization possibilities as a source of phenolic compounds. J. Supercrit. Fluids 2014, 92, 55–59. [Google Scholar] [CrossRef]
- Jimenez-Aguilar, D.M.; Escobedo-Avellaneda, Z.; Martin-Belloso, O.; Gutierrez-Uribe, J.; Valdez-Fragoso, A.; Garcia-Garcia, R.; Torres, J.A.; Welti-Chanes, J. Effect of high hydrostatic pressure on the content of phytochemical compounds and antioxidant activity of prickly pears (Opuntia ficus-indica) beverages. Food Eng. Rev. 2015, 7, 198–208. [Google Scholar] [CrossRef]
- Lee, D.; Ghafoor, K.; Moon, S.; Kim, S.H.; Kim, S.; Chun, H.; Park, J. Phenolic compounds and antioxidant properties of high hydrostatic pressure and conventionally treated ginseng (Panax ginseng) products. Qual. Assur. Saf. Crops Foods 2015, 7, 493–500. [Google Scholar] [CrossRef]
- Teh, S.; Niven, B.E.; Bekhit, A.E.A.; Carne, A.; Birch, E.J. Microwave and pulsed electric field assisted extractions of polyphenols from defatted canola seed cake. Int. J. Food Sci. Technol. 2015, 50, 1109–1115. [Google Scholar] [CrossRef]
- Segovia, F.J.; Luengo, E.; Corral-Perez, J.J.; Raso, J.; Pilar Almajano, M. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crop. Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- Luengo, E.; Alvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Rosello-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioprocess Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Esclapez, M.D.; Garcia-Perez, J.V.; Mulet, A.; Carcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A comparative analysis of the “green” techniques applied for polyphenols extraction from bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
- Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.C.G.; Chemat, F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochem. 2010, 17, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Wu, C.Y.; Weng, Y.M.; Tseng, C.Y. Ultrasound-assisted extraction methodology as a tool to improve the antioxidant properties of herbal drug Xiao-chia-hu-tang. J. Ethnopharmacol. 2005, 99, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Hamid, A.A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011, 18, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Zhai, J.W.; Cui, Q.; Liu, J.Z.; Luo, M.; Fu, Y.J.; Zu, Y.G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Tan, Z.J.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Wang, C.Y. Extraction, preconcentration and isolation of flavonoids from Apocynum venetum L. Leaves using ionic liquid-based ultrasonic-assisted extraction coupled with an aqueous biphasic system. Molecules 2016, 21, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Florez, N.; Conde, E.; Dominguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Pap, N.; Beszedes, S.; Pongracz, E.; Myllykoski, L.; Gabor, M.; Gyimes, E.; Hodur, C.; Keiski, R.L. Microwave-assisted extraction of anthocyanins from black currant marc. Food Bioprocess Technol. 2013, 6, 2666–2674. [Google Scholar] [CrossRef]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. Leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Pasrija, D.; Anandharamakrishnan, C. Techniques for extraction of green tea polyphenols: A review. Food Bioprocess Technol. 2015, 8, 935–950. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC-Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ding, L.; Li, T.C.; Zhou, X.; Wang, L.; Zhang, H.Q.; Liu, L.; Li, Y.; Liu, Z.H.; Wang, H.J.; et al. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim. J. Chromatogr. A 2006, 1102, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S. A novel technology for extraction of phenolic antioxidants from mandarin (Citrus deliciosa Tenore) leaves: Solvent-free microwave extraction. Korean J. Chem. Eng. 2015, 32, 950–957. [Google Scholar] [CrossRef]
- Bendjersi, F.Z.; Tazerouti, F.; Belkhelfa-Slimani, R.; Djerdjouri, B.; Meklati, B.Y. Phytochemical composition of the Algerian Laurus nobilis L. Leaves extracts obtained by solvent-free microwave extraction and investigation of their antioxidant activity. J. Essent. Oil Res. 2016, 28, 202–210. [Google Scholar] [CrossRef]
- Zill-e-Huma, M.A.V.; Maingonnat, J.F.; Chemat, F. Clean recovery of antioxidant flavonoids from onions: Optimising solvent free microwave extraction method. J. Chromatogr. A 2009, 1216, 7700–7707. [Google Scholar] [CrossRef] [PubMed]
- Perino-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Elfakir, C. Evaluation of a simple and promising method for extraction of antioxidants from sea buckthorn (Hippophae rhamnoides L.) berries: Pressurised solvent-free microwave assisted extraction. Food Chem. 2011, 126, 1380–1386. [Google Scholar] [CrossRef]
- Gu, H.; Chen, F.; Zhang, Q.; Zang, J. Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1014, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Bi, L.W.; Zhao, Z.D.; Chen, Y.X. Advances in enzyme assisted extraction of natural products. In AER-Advances in Engineering Research, 3nd ed.; Yarlagadda, P., Ed.; Atlantis Press: Paris, France, 2015; Volume 27, pp. 371–375. [Google Scholar]
- Liu, X.; Hu, Y.; Wei, D. Optimization of enzyme-based ultrasonic/microwave-assisted extraction and evaluation of antioxidant activity of orcinol glucoside from the rhizomes of Curculigo orchioides Gaertn. Med. Chem. Res. 2014, 23, 2360–2367. [Google Scholar] [CrossRef]
- Dinkova, R.; Heffels, P.; Shikov, V.; Weber, F.; Schieber, A.; Mihalev, K. Effect of enzyme-assisted extraction on the chilled storage stability of bilberry (Vaccinium myrtillus L.) anthocyanins in skin extracts and freshly pressed juices. Food Res. Int. 2014, 65, 35–41. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Smagghe, G.; Gonzales, G.B.; van Camp, J.; Raes, K. Enzyme-assisted extraction enhancing the phenolic release from cauliflower (Brassica oleracea L. Var. Botrytis) outer Leaves. J. Agric. Food Chem. 2014, 62, 7468–7476. [Google Scholar]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol.-Mysore 2015, 52, 5048–5056. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, S.; Lee, D.; Park, D.J.; Imm, J. Enzyme and high pressure assisted extraction of tricin from rice hull and biological activities of rice hull extract. Food Sci. Biotechnol. 2016, 25, 159–164. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; del Pilar Sanchez-Camargo, A.; Cifuentes, A.; Ibanez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC-Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of phenolic compounds from rice (Oryza sativa) grains. Food Chem. 2016, 192, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E. Simultaneous HPLC determination of carotenoids used as food coloring additives: Applicability of accelerated solvent extraction. Food Chem. 2004, 86, 449–456. [Google Scholar] [CrossRef]
- Bozan, B.; Altinay, R.C. Accelerated solvent extraction of flavan-3-ol derivatives from grape seeds. Food Sci. Technol. Res. 2014, 20, 409–414. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marce, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. TrAC Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Franquin-Trinquier, S.; Maury, C.; Baron, A.; Le Meurlay, D.; Mehinagic, E. Optimization of the extraction of apple monomeric phenolics based on response surface methodology: Comparison of pressurized liquid-solid extraction and manual-liquid extraction. J. Food Compos. Anal. 2014, 34, 56–67. [Google Scholar] [CrossRef]
- Pineiro, Z.; Palma, M.; Barroso, C.G. Determination of trans-resveratrol in grapes by pressurised liquid extraction and fast high-performance liquid chromatography. J. Chromatogr. A 2006, 1110, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Typek, R.; Dawidowicz, A.L. Chlorogenic acid stability in pressurized liquid extraction conditions. J. AOAC Int. 2015, 98, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Yang, S.; Chen, C.; Jesisca; Chang, J. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-solvent selection for supercritical fluid extraction of astaxanthin and other carotenoids from Penaeus monodon Waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC-Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Konar, N.; Dalabasmaz, S.; Poyrazoglu, E.S.; Artik, N.; Colak, A. The determination of the caffeic acid derivatives of Echinacea purpurea aerial parts under various extraction conditions by supercritical fluid extraction (SFE). J. Supercrit. Fluids 2014, 89, 128–136. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. Mysore 2014, 51, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.F.; Konat Gandolfi, P.H.; Almeida, R.N.; Lucas, A.M.; Cassel, E.; Figueiro Vargas, R.M. Analysis of supercritical fluid extraction of lycopodine using response surface methodology and process mathematical modeling. Chem. Eng. Res. Des. 2015, 100, 353–361. [Google Scholar] [CrossRef]
- Haghayegh, M.; Zabihi, F.; Eikani, M.H.; Moghadas, B.K.; Yazdi, S.A.V. Supercritical fluid extraction of flavonoids and terpenoids from herbal compounds: Experiments and mathematical modeling. J. Essent. Oil Bear. Plants. 2015, 18, 1253–1265. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Framboisier, X.; Frochot, C.; Vanderesse, R.; Barth, D.; Kalthoum-Cherif, J.; Blanchard, F.; Guiavarc’h, Y. Response surface methodology applied to supercritical fluid extraction (SFE) of carotenoids from Persimmon (Diospyros kaki L.). Food Chem. 2016, 208, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, D.; Liu, J.; Xu, K.; Wu, G. Modeling of supercritical fluid extraction of flavonoids from Calycopteris floribunda leaves. Chem. Pap. 2014, 68, 316–323. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. Supercritical fluids and ultrasound assisted extractions applied to spruce bark conversion. Environ. Eng. Manag. J. 2015, 14, 615–623. [Google Scholar]
- Solana, M.; Mirofci, S.; Bertucco, A. Production of phenolic and glucosinolate extracts from rocket salad by supercritical fluid extraction: Process design and cost benefits analysis. J. Food Eng. 2016, 168, 35–41. [Google Scholar] [CrossRef]
- Pasquel Reategui, J.L.; Da Fonseca Machado, A.P.; Barbero, G.F.; Rezende, C.A.; Martinez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Corrales, M.; Garcia, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Altuner, E.M.; Tokusoglu, O. The effect of high hydrostatic pressure processing on the extraction, retention and stability of anthocyanins and flavonols contents of berry fruits and berry juices. Int. J. Food Sci. Technol. 2013, 48, 1991–1997. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Xi, J.; Shen, D.; Zhao, S.; Lu, B.; Li, Y.; Zhang, R. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm. 2009, 382, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, V.D.; Putnik, P.; Zoric, Z.; Jezek, D.; Karlovic, S.; Kovacevic, D.B. Winery by-products: Anthocyanins recovery from red grape skin by high hydrostatic pressure extraction (HHPE). Ann. Nutr. Metab. 2015, 671, 522–523. [Google Scholar]
- Kovacevic, D.B.; Putnik, P.; Pedisic, S.; Jazek, D.; Karlovic, S.; Uzelac, V.D. High hydrostatic pressure extraction of flavonoids from freeze-dried red grape skin as winemaking by-product. Ann. Nutr. Metab. 2015, 671, 521–522. [Google Scholar]
- Xi, J.; Luo, S. The mechanism for enhancing, extraction of ferulic acid from Radix Angelica sinensis by high hydrostatic pressure. Sep. Purif. Technol. 2016, 165, 208–213. [Google Scholar] [CrossRef]
- Lopez, N.; Puertolas, E.; Condon, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- Zderic, A.; Zondervan, E. Polyphenol extraction from fresh tea leaves by pulsed electric field: A study of mechanisms. Chem. Eng. Res. Des. 2016, 109, 586–592. [Google Scholar] [CrossRef]
- Bouras, M.; Grimi, N.; Bals, O.; Vorobiev, E. Impact of pulsed electric fields on polyphenols extraction from Norway spruce bark. Ind. Crop. Prod. 2016, 80, 50–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Huang, H. Effects of pulsed electric fields on anthocyanin extraction yield of blueberry processing by-products. J. Food Process Preserv. 2015, 39, 1898–1904. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L. Optimization and characterization of pulsed electric field parameters for extraction of quercetin and ellagic acid in emblica officinalis juice. J. Food Meas. Charact. 2014, 8, 225–233. [Google Scholar] [CrossRef]
- Gachovska, T.; Cassada, D.; Subbiah, J.; Hanna, M.; Thippareddi, H.; Snow, D. Enhanced anthocyanin extraction from red cabbage using pulsed electric field processing. J. Food Sci. 2010, 75, E323–E329. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Martinez, J.M.; Bordetas, A.; Alvarez, I.; Raso, J. Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Roohinejad, S.; Oey, I.; Everett, D.W.; Niven, B.E. Evaluating the effectiveness of beta-carotene extraction from pulsed electric field-treated carrot pomace using oil-in-water microemulsion. Food Bioprocess Technol. 2014, 7, 3336–3348. [Google Scholar] [CrossRef]
- Lopez, N.; Puertolas, E.; Condon, S.; Alvarez, I.; Raso, J. Effects of pulsed electric fields on the extraction of phenolic compounds during the fermentation of must of Tempranillo grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Boussetta, N.; Lanoiselle, J.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Electrical, mechanical, and chemical effects of high-voltage electrical discharges on the polyphenol extraction from vine shoots. Innov. Food Sci. Emerg. Technol. 2015, 31, 60–66. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Ferreira Marczak, L.D.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Le, H.V.; Le, V.V.M. Comparison of enzyme-assisted and ultrasound-assisted extraction of vitamin C and phenolic compounds from acerola (Malpighia emarginata DC.) fruit. Int. J. Food Sci. Technol. 2012, 47, 1206–1214. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Gilbert-Lopez, B.; Mendiola, J.A.; Quirantes-Pine, R.; Segura-Carretero, A.; Ibanez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Mandal, V.; Mandal, S.C. A brief understanding of process optimisation in microwave-assisted extraction of botanical materials: Options and opportunities with chemometric tools. Phytochem. Anal. 2014, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Eskilsson, C.S.; Bjorklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Senorans, F.J.; Ibanez, E.; Cifuentes, A. New trends in food processing. Crit. Rev. Food Sci. Nutr. 2003, 43, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Arts, M.; Dallinga, J.S.; Voss, H.P.; Haenen, G.; Bast, A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays (Reprinted). J. Funct. Food. 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Wu, X.L.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.X.; Huang, D.J.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Ozyurek, M.; Guclu, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC-Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Demirata, B.; Ozyurek, M.; Celik, S.E.; Bektasoglu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Elsevier Academic Press: Sandiego, Chile, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C.; Toth, I.V.; Rangel, A.O.S.S. Automatic flow system for sequential determination of ABTS(center dot+) scavenging capacity and Folin-Ciocalteu index: A comparative study in food products. Anal. Chim. Acta 2007, 592, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghia, M.; Manzoori, J.L.; Jouyban, A. Determination of total phenols in tea infusions, tomato and apple juice by terbium sensitized fluorescence method as an alternative approach to the Folin-Ciocalteu spectrophotometric method. Food Chem. 2008, 108, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, E.J.; Leskovar, D.; Patil, B.S. Development of an automated method for Folin-Ciocalteu total phenolic assay in artichoke extracts. J. Food Sci. 2012, 77, C1278–C1283. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Olgun, F.A.O.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Nkhili, E.; Brat, P. Reexamination of the ORAC assay: Effect of metal ions. Anal. Bioanal. Chem. 2011, 400, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Gibson, P.G.; Garg, M.L. A review of the methodology for assessing in vivo antioxidant capacity. J. Sci. Food Agric. 2006, 86, 2057–2066. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, G.; Zhang, Q.; Liu, X.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Determining antioxidant activities of lactobacilli by cellular antioxidant assay in mammal cells. J. Funct. Food 2015, 19, 554–562. [Google Scholar] [CrossRef]
- Blasa, M.; Angelino, D.; Gennari, L.; Ninfali, P. The cellular antioxidant activity in red blood cells (CAA-RBC): A new approach to bioavailability and synergy of phytochemicals and botanical extracts. Food Chem. 2011, 125, 685–691. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xu, Y.; Zhang, T.; Lu, J.J. Assessing plant antioxidants by cellular antioxidant activity assay based on microfluidic cell chip with arrayed microchannels. Chin. J. Anal. Chem. 2016, 44, 604–609. [Google Scholar] [CrossRef]
- O’sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanniffy, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Baroni, M.V.; Di Paola Naranjo, R.D.; Garcia-Ferreyra, C.; Otaiza, S.; Wunderlin, D.A. How good antioxidant is the red wine? Comparison of some in vitro and in vivo methods to assess the antioxidant capacity of Argentinean red wines. LWT-Food Sci. Technol. 2012, 47, 1–7. [Google Scholar] [CrossRef]
- Biswas, S.K.; McClure, D.; Jimenez, L.A.; Megson, I.L.; Rahman, I. Curcumin induces glutathione biosynthesis and inhibits NF-κB activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005, 7, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-alpha and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular antioxidant activity of common vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Surarit, W.; Jansom, C.; Lerdvuthisopon, N.; Kongkham, S.; Hansakul, P. Evaluation of antioxidant activities and phenolic subtype contents of ethanolic bran extracts of Thai pigmented rice varieties through chemical and cellular assays. Int. J. Food Sci. Technol. 2015, 50, 990–998. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [PubMed]
- Mezzomo, N.; Ferreira, S.R. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Borguini, R.G.; Ferraz Da Silva Torres, E.A. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.D.J.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lu, W.; Niu, Y.; Liu, J.; Zhang, X.; Gao, B.; Akoh, C.C.; Shi, H.; Yu, L.L. Identification and quantification of phytochemical composition and anti-inflammatory, cellular antioxidant, and radical scavenging activities of 12 plantago species. J. Agric. Food Chem. 2013, 61, 6693–6702. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Wang, H.; Guo, X.; Abbasi, A.M.; Wang, T.; Li, T.; Fu, X.; Li, J.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of seven cultivars of Aloe. Int. J. Food Sci. Technol. 2016, 51, 1489–1494. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.Y.; Yu, X.Y.; Liu, D.; Wan, H.X. Evaluation of cellular antioxidant and antiproliferative activities of five main Phyllanthus emblica L. cultivars in China. Indian J. Pharm. Sci. 2015, 77, 274–282. [Google Scholar] [CrossRef] [PubMed]

| Source | Compounds Extracted | Extraction Parameters | Extraction Improvement | Reference | |
|---|---|---|---|---|---|
| Non-Conventional Method | Conventional Methods | ||||
| ultrasound-assisted extraction (UAE) | |||||
| blueberry wine pomace | anthocyanins and phenolics | solvents: 70% ethanol and 0.01% hydrochloric acid; conditions: 400 w, 61.03 °C, 23.67 min | 70% ethanol and 0.01% hydrochloric acid; 61 °C, 35 min without ultrasound treatment | increased total anthocyanins from 1.72 to 4.27 mg C3G/g (2.5-fold) and total phenolics from 5.08 to 16.41 mg gallic acid equivalent (GAE)/g (about 3.2-fold) | [42] |
| papaya | lycopene | solvents: 42.28% ethanol in ethyl acetate conditions: 40 kHz, 800 W, 26.09 min, 50.12 °C | 40% ethanol in ethyl acetate (300 mL) 95 °C in a Soxhlet extractor | Recovery of lycopene increased from 68.3 ± 4.1 to 189.8 ± 4.5 μg/g | [43] |
| carrot | carotenoids | solvents: sunflower oil conditions: 22.5 W/cm2, 40 °C, 20 min | hexane at room temperature for one hour | obtained the β-carotene yield of 334.75 mg/L just in 20 min while the CSE method using hexane as solvent obtained the β-carotene yield of 321.36 mg/L after one-hour extraction | [44] |
| microwave-assisted extraction (MAE) | |||||
| Achillea millefolium dust | antioxidants | solvents: 70% ethanol conditions: 170 W, 40 mL/g, 33 s | 40% ethanol at room temperature (1:10, v/v) for 48 h | increased total polyphenol content from 135.26 ± 1.72 to 237.74 ± 2.08 mg GAE/g, total flavonoid content from 30.82 ± 2.35 to 42.95 ± 1.32 mg quercetin equivalents (QE)/g, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity from 21.58% ± 0.88% to 71.72% ± 2.12% | [45] |
| Quercus bark | polyphenols | solvents: ethanol content 33%, methanol content 0.38% conditions: 50 Hz, 45 W, 60 min, pH 10.75, room temperature | the same extraction condition without microwave treatment | increased by 3 times and 2 times respectively for total phenolic content and antioxidant recoveries | [46] |
| enzyme-assisted extraction (EAE) | |||||
| wine making by-products | phenolics | solvents: 70% acetone enzyme treatment with 2% viscozyme solution stirred for 12 h at 37 °C or 1 mg/mL pronase solution stirred for 1 h, then extraction with 70% (v/v) acetone in a gyratory water bath shaker at 30 °C for 20 min | the same extraction protocol without enzyme treatment | pronase and viscozyme increased the content of soluble phenolics while reducing the content of insoluble-bound phenolics | [32] |
| grape skins | flavonoids | solvents: buffer solution containing an appropriate amount of enzyme conditions: 10.52 mg/g Lallzyme EX-V, pH 2.0, extraction at 45 °C for 3 h | 70% aqueous ethanol containing 1% formic acid for one day in the dark | improved recovery of anthocyanin contents (from 40,496.19 ± 58.18 to 41,752.95 ± 76.10 mg/kg) and flavan-3-ol contents (from 329.32 ± 2.46 to 345.94 ± 2.88 mg/kg) | [47] |
| tomato processing waste | lycopene | solvents: hexane/acetone/ethanol (50:25:25 v/v) conditions: 1.5% cellulase/2% pectinase at 4 h of incubation period | without enzyme treatment | increased the yield of lycopene from less than 200 to 847.33 μg/g (cellulase treatment) and to 1262.56 μg/g (pectinase treatment) | [48] |
| pressurized liquid extraction (PLE) | |||||
| Aerial parts of Dracoceph-alum kotschyi | phenolics and flavonoids | solvents: methanol conditions: 74 °C, 34 bar pressure, 11.33 min static time, 17.45 min dynamic time, and 0.7 mL/min solvent flow rate | percolated with 1.0 L of methanol at room temperature (25 °C) according to the European Pharmacopeia | improved recovery of total phenolic (from 22.29 ± 0.05 to 30.92 ± 0.03 GAE mg/g), total flavonoids (from 5.042 ± 0.04 to 6.13 ± 0.07 QE mg/g) and luteolin content (from 9550 ± 0.3 to 13,247 ± 0.2 μg/g) | [49] |
| roots of Scutellaria pinnatifida | phenolics and flavonoids | solvents: methanol conditions: 65.8 °C, 39.2 bar pressure, 12.9 min static time, 18.9 min dynamic time, and 0.76 mL/min solvent flow rate | percolated with 1.0 L of methanol at room temperature | the total phenolic content increased from 196.66 to 396.94 mg/g, and the total flavonoid content increased from 91.3 to 127.78 mg/g | [50] |
| black bamboo leaves | antioxidants | solvents: 50% ethanol for the total phenolic (TP) and 75% ethanol for total flavonoid (TF) and 25% ethanol for DPPH radical scavenging ability conditions: 1500 psi, 200 °C, 25 min static time | reflux extraction method (~90 °C, 1 L solvent, 60 min) | improved extraction yields from 240 to 500 mg/1 g Dry black bamboo leaves (DL), TP contents from 1510 ± 3.2 to 2682 ± 0.9 mg/100 g, TF contents from 182 ± 2.7 to 657 ± 1.7 mg/100 g | [51] |
| palm pressed fiber | β-carotene | solvents: n-hexane conditions: 80 °C, 1500 psi, 2 × 10 min static extractions with flush volume 50% | extracted with n-hexane and chloroform in a Soxhlet apparatus for 8 h | obtained total β-carotene and vitamin contents comparable to Soxhlet extraction but with lower total organic solvent and rapid extraction process | [52] |
| supercritical fluid extraction (SFE) | |||||
| myrtle leaves and berries | antioxidants | solvents: carbon dioxide conditions: 23 MPa, 45 °C and a CO2 flow of 0.3 kg/h using absolute ethanol as co-solvent with a flow rate of 0.09 kg/h | obtained by hydrodistillation using a Clevenger-type apparatus, for two hours | increased antioxidant capacity (about 20–40 times), polyphenolic contents (about 2 times) and myricetin-3-O-rhamnoside content (about 110–170 times in fruit and about 130–210 times in leaves) | [53] |
| Prunus persica leaves | phenolic compounds | solvents: carbon dioxide conditions: 60 °C, 150 bar and 6% ethanol co-solvent at a flow rate of 15 g/min and for a duration of 60 min | extracted 3 times with 30 mL of solvent system (acetone:methanol:water:formic acid, 40:40:20:0.1) | the radical scavenging activity value increased from 32.23% to 53.25% | [54] |
| high hydrostatic pressure extraction (HHPE) | |||||
| prickly pear beverages prepared with 10% peel and 90% pulp | Phyto-chemical Compounds | 400 or 550 MPa, room temperature, 0–16 min | thermally treated at 138 ± 1 °C for 2 s | increased TP content (16%–35%) and antioxidant activity (8%–17%) for Cristal (A) and Rojo San Martin varieties as well as increased the betaxanthin contents (6%–8%) and betacyanin content (4%–7%) for Rojo San Martin variety | [55] |
| Panax ginseng | phenolic compounds | 600 MPa for 1 min at room temperature | conventional steaming | increased the total phenolic contents (from 1.13 to 1.37 mg maltol equivalent/g of red ginseng), especially maltol content (4.38 to 12.61 mg/100 g of red ginseng), also improved the ferrous ion chelating and superoxide dismutase activities | [56] |
| Pulsed electric field extraction (PEFE) | |||||
| defatted canola seed cake | polyphenols | 10% ethanol 30 V, 30 Hz and 10 s | microwave processing (5 min, liquid/solid ratio of 6.0 and 633.3 W) | less solvent usage, a shorter extraction time | [57] |
| Borago officinalis L. leaves | polyphenols | acidic water (pH 1.5) 1 to 7 kV/cm, 15–150 μs, 0.04 to 61.1 kJ/kg | the same extraction without PEF treatment | increased the TPC (1.3–6.6 times) and ORAC values (2.0–13.7 times) | [58] |
| orange peel | polyphenols | distilled water 5 kV/cm, 60 μs, 0.06 to 3.77 kJ/kg, pressurization at 5 bars for 30 min | the same extraction without PEF treatment | increased the naringin content from 1 to 3.1 mg/100 g and hesperidin content from 1.3 to 4.6 mg/100 g | [59] |
| high voltage electrical discharges extraction (HVEDE) | |||||
| olive kernel | phenolic compounds | 49% ethanol, 66 kJ/kg, pH 2.5 | PEF with electric field strength E = 13.3 kV/cm and UAE at 400 W and 24 kHz | more effective polyphenol extraction (255 mg GAE/L for HVEDE versus 140 and 146 mg GAE/L for UAE and PEFE, respectively) | [60] |
| Method | Brief Description | Investment Cost | Energy Efficiency | Merits | Drawbacks | Reference |
|---|---|---|---|---|---|---|
| ultrasound-assisted extraction | Sample is extracted with solvent in a vessel and immersed in an ultrasonication bath. | low | medium | fast energy transfer; high extraction yield; low solvent use; short extraction time (5–60 min) | lack of uniformity in the process; generating damages to the ear of the operator; filtration and clean-up step required. | [64,65,144,145] |
| microwave -assisted extraction | Sample is extracted with a microwave absorbing solvent in a closed/open vessel and irradiated with microwave. | medium | medium | quick heating for bioactive compounds extraction; high extraction yield; low solvent use; moderate extraction time (1 min–40 min) | Extraction solvent must be able to absorb microwaves; filtration and cleanup step required. | [64,75,145] |
| enzyme-assisted extraction | Sample and enzyme solution are loaded into a vessel and placed in a water bath thermostated at the certain temperature and time. | medium | medium | moderate extraction conditions; eco-friendly; selectivity due to the specificity of enzymes | Expensive cost of enzymes; activity of enzymes varying with the environmental factors; filtration and cleanup step required. | [88,91,94] |
| pressurized liquid extraction | Sample and solvent are heated and pressurized in a vessel with elevated temperature and pressure. After finishing the extraction, the extract is automatically into a vial. | high | high | high extraction yield; low time and solvent consumption; protection for oxygen and light sensitive compounds; no filtration required; automated systems | clean-up step required; expensive equipment required. | [96,145] |
| supercritical fluid extraction | Sample is extracted with super-critical fluid in a vessel with high pressure. the analytes are collected in a small volume of solvent or onto a solid-phase trap. | high | high | green solvents (e.g., CO2) used; high extraction yield; better separation of solute from solvent; possibility to on-line combining with chromato-graphic process; reduced the thermal degradation; no cleanup or filtration required; automated systems | limited ability to dissolve polar compounds; more parameters to optimize. | [64,106,107,117,145] |
| high hydrostatic pressure extraction | Sample was mixed with solvent and placed in a sterile poly-ethylene bag, which is eliminated air from the inside and placed into a pressure extractor at different pressure values. | high | high | waste-free process; short time (only about 5 min); performed at room temperature without any heating process | High investment cost and cost-intensive maintenance and service, which make industrial application difficult. | [123,127,146] |
| pulsed electric field extraction | Extraction was performed between two plate electrodes with 2–3 cm distance and the sample is placed in the treatment chamber. | high | high | mild (non-thermal) processing method; short time (less than 1 s) | Extraction must be applied to food products that can withstand high electric fields and have low electrical conductivity. | [129,146,147] |
| high voltage electrical discharges extraction | HVED treatment was performed between the stainless steel needle and the grounded plate electrodes with 1 cm distance and the sample is placed in the treatment chamber. | high | high | mild (non-thermal) processing method; high extraction efficiency; short extraction time | High voltage electrical discharges may generate chemical products and free reactive radicals, which can react with antioxidant compounds, thus decreasing their bioactive activity. | [147] |
| Category | Varieties Showing Strong Antioxidant Activities | Assessment Method | Reference |
|---|---|---|---|
| antioxidant activities at chemical level | |||
| 26 spices | oregano, cinnamon stick, clove, cinnamon, sage | Trolox equivalent antioxidant capacity (TEAC), Folin–Ciocalteu reagent (FCR) | [8] |
| 62 fruits | Chinese date, pomegranate, guava, sweetsop, persimmon, Chinese wampee and plum, grape (red) | TEAC, ferric-reducing antioxidant power (FRAP), FCR | [11] |
| 24 cereal grains | black rice, red rice, purple rice, buckwheat | TEAC, FRAP, FCR | [12] |
| 49 Edible macro-fungi | Thelephora ganbajun, Boletus edulis, Volvariella volvacea, Boletus regius, and Suillus bovinus | TEAC, FRAP, FCR | [13] |
| 56 vegetables | Chinese toon Bud, loosestrife, perilla leaf, cowpea, caraway, lotus root, sweet potato leaf, soy bean (green), pepper leaf, ginseng leaf, chives, and broccoli | TEAC, FRAP, FCR | [14] |
| 223 medicinal plants | Acanthopanax gracilistylus, Agrimonia pilosa, Anemarrhena asphodeloides, Caesalpina sappan, Carthamus tinctorius, Dioscorea bulbifera, Fraxinus rhynchophylla, Lonicera japonica (flower), Magnolia officinalis, Mentha haplocalyx, Paeonia lactiflora (red), Polygonum multiflorum (Stem), Polygonum multiflorum (Root), Rhodiola sacra, Salvia miltiorrhiza, Tussilago farfara, Sargentodoxa cuneata | TEAC, FRAP, FCR | [15] |
| 51 edible and wild flowers | Rosa rugosa, Limonium sinuatum, Pelargonium hortorum, Jatropha integerrima and Osmanthus fragrans, Orostachys fimbriatu, Chaenomeles sinensis, Calliandra haematocephala | TEAC, FRAP, FCR | [16] |
| 50 fruit wastes | grape seed, hawthorn peel, longan peel, longan seed, mango peel, Chinese olive peel and sweetsop peel | TEAC, FRAP, FCR | [19] |
| antioxidant activities at cellular level | |||
| 27 vegetables | beet, broccoli, and red pepper, eggplant, Brussels sprout, cabbage | cellular antioxidant activity (CAA) based on HepG2 cells | [183] |
| 25 fruits | pomegranate and berries (wild blueberry, blackberry, raspberry, and blueberry) | CAA based on HepG2 cells | [184] |
| 11 Thai pigmented (red and purple) and 2 nonpigmented rice varieties | hawm dowk mali deang (red), hawm deang sukhothai1 (red), hawm deang (red), man pu (red), red rose (red), klam moang (purple), klam chiang mai (purple) | CAA based on HL-60 cells | [185] |
| 13 food legumes | black soybean, black bean, pinto bean, lentil, green pea, yellow soybean | CAA based on human gastric adenocarcinoma AGS cells | [192] |
| 12 plantago species | P. lanceolata, P. himalaica, P. depressa, P. cornuti, P. jehohlensis | CAA based on HepG2 cells | [193] |
| seven cultivars of Aloe | Aloe. greenii, Aloe. arborescens | CAA based on HepG2 cells | [194] |
| five main Phyllanthus emblica L. cultivars | qingyougan, binggan and boligan | CAA based on HepG2 cells | [195] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010096
Xu D-P, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang J-J, Li H-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. International Journal of Molecular Sciences. 2017; 18(1):96. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010096
Chicago/Turabian StyleXu, Dong-Ping, Ya Li, Xiao Meng, Tong Zhou, Yue Zhou, Jie Zheng, Jiao-Jiao Zhang, and Hua-Bin Li. 2017. "Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources" International Journal of Molecular Sciences 18, no. 1: 96. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18010096