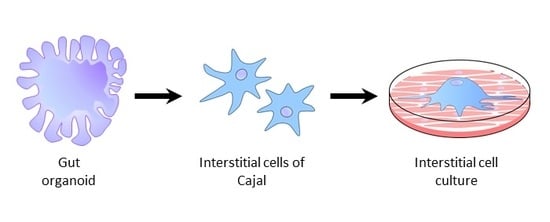
The Potential for Gut Organoid Derived Interstitial Cells of Cajal in Replacement Therapy
Abstract
:1. Introduction
2. ICC Description
3. Generation of Gut Organoids and ICC
4. Current ICC Makers
5. Purification and Cell-Sorting Methods
6. Functional Analysis
7. ICC Transplantation and Regeneration
8. Integration
9. Conclusions and Prospects
Author Contributions
Conflicts of Interest
Abbreviations
| ICC | Interstitial cells of Cajal |
| GI | Gastrointestinal tract |
| MY | Myenteric plexus |
| LM | Longitudinal muscles |
| DMP | Deep muscular plexus |
| iPSC | Induced pluripotent stem cells |
| ENS | Enteric nervous system |
References
- Choi, R.S.; Vacanti, J.P. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant. Proc. 1997, 29, 848–851. [Google Scholar] [CrossRef]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Machida, M.; Miura, T.; Kawasaki, T.; Okazaki, T.; Sasaki, K.; Sakamoto, S.; Ohuchi, N.; Kasahara, M.; Umezawa, A.; et al. A xenogeneic-free system generating functional human gut organoids from pluripotent stem cells. JCI Insight 2017, 2, e86492. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 1996, 111, 492–515. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Koh, S.D.; Ward, S.M. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 2006, 68, 307–343. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.D. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: In vitro versus in vivo studies. J. Cell. Mol. Med. 2008, 12, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Ordog, T.; Ward, S.M.; Sanders, K.M. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J. Physiol. 1999, 518, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Szurszewski, J.H. Electrical basis for gastrointestinal motility. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Raven: New York, NY, USA, 1987; pp. 383–422. [Google Scholar]
- Cousins, H.M.; Edwards, F.R.; Hickey, H.; Hill, C.E.; Hirst, G.D. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J. Physiol. 2003, 550, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Berezin, I.; Huizinga, J.D.; Daniel, E.E. Structural characterization of interstitial cells of Cajal in myenteric plexus and muscle layers of canine colon. Can. J. Physiol. Pharmacol. 1990, 68, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Thuneberg, L. Interstitial cells of Cajal: Intestinal pacemaker cells? Adv. Anat. Embryol. Cell. Biol. 1982, 71, 1–130. [Google Scholar] [PubMed]
- Burns, A.J.; Lomax, A.E.; Torihashi, S.; Sanders, K.M.; Ward, S.M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl. Acad. Sci. USA 1996, 93, 12008–12013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Buxton, I.L. Muscarinic receptors in canine colonic circular smooth muscle. II. Signal transduction pathways. Mol. Pharmacol. 1991, 40, 952–959. [Google Scholar] [PubMed]
- Daniel, E.E.; Posey-Daniel, V. Neuromuscular structures in opossum esophagus: Role of interstitial cells of Cajal. Am. J. Physiol. 1984, 246, G305–G315. [Google Scholar] [PubMed]
- Wang, X.Y.; Sanders, K.M.; Ward, S.M. Intimate relationship between interstitial cells of cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999, 295, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Beckett, E.A.; Horiguchi, K.; Khoyi, M.; Sanders, K.M.; Ward, S.M. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J. Physiol. 2002, 543, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Cortesini, C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J. Submicrosc. Cytol. 1985, 17, 673–685. [Google Scholar] [PubMed]
- Gockel, I.; Bohl, J.R.; Eckardt, V.F.; Junginger, T. Reduction of interstitial cells of Cajal (ICC) associated with neuronal nitric oxide synthase (n-NOS) in patients with achalasia. Am. J. Gastroenterol. 2008, 103, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lyford, G.L.; He, C.L.; Soffer, E.; Hull, T.L.; Strong, S.A.; Senagore, A.J.; Burgart, L.J.; Young-Fadok, T.; Szurszewski, J.H.; Farrugia, G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 2002, 51, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Wedel, T.; Spiegler, J.; Soellner, S.; Roblick, U.J.; Schiedeck, T.H.; Bruch, H.P.; Krammer, H.J. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology 2002, 123, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, K.; Hirota, S.; Miyagawa, J.; Taniguchi, M.; Shinomura, Y.; Matsuzawa, Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am. J. Gastroenterol. 1997, 92, 332–334. [Google Scholar] [PubMed]
- Kenny, S.E.; Vanderwinden, J.M.; Rintala, R.J.; Connell, M.G.; Lloyd, D.A.; Vanderhaegen, J.J.; De Laet, M.H. Delayed maturation of the interstitial cells of Cajal: A new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J. Pediatr. Surg. 1998, 33, 94–98. [Google Scholar] [CrossRef]
- Porcher, C.; Baldo, M.; Henry, M.; Orsoni, P.; Jule, Y.; Ward, S.M. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Rumessen, J.J. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology 1996, 111, 1447–1455. [Google Scholar] [CrossRef]
- Lu, G.; Qian, X.; Berezin, I.; Telford, G.L.; Huizinga, J.D.; Sarna, S.K. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am. J. Physiol. 1997, 273, G1233–G1245. [Google Scholar] [PubMed]
- He, C.L.; Soffer, E.E.; Ferris, C.D.; Walsh, R.M.; Szurszewski, J.H.; Farrugia, G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 2001, 121, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Kajimura, M.; Osawa, S.; Kanaoka, S.; Furuta, T.; Ikuma, M.; Hishida, A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J. Gastroenterol. 2006, 41, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Ordog, T.; Takayama, I.; Cheung, W.K.; Ward, S.M.; Sanders, K.M. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 2000, 49, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Ro, S.; Park, C.; Jin, J.; Zheng, H.; Blair, P.J.; Redelman, D.; Ward, S.M.; Yan, W.; Sanders, K.M. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology 2010, 138, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.; Redelman, D.; Horvath, V.J.; Bardsley, M.R.; Chen, H.; Ordog, T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology 2008, 134, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Epperson, A.; Hatton, W.J.; Callaghan, B.; Doherty, P.; Walker, R.L.; Sanders, K.M.; Ward, S.M.; Horowitz, B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am. J. Physiol. Cell Physiol. 2000, 279, C529–C539. [Google Scholar] [PubMed]
- Edwards, F.R.; Hirst, G.D.; Suzuki, H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J. Physiol. 1999, 519, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Won, K.J.; Sanders, K.M.; Ward, S.M. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc. Natl. Acad. Sci. USA 2005, 102, 14913–14918. [Google Scholar] [CrossRef] [PubMed]
- Iino, S.; Ward, S.M.; Sanders, K.M. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J. Physiol. 2004, 556, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Semple, G.S.; Sanders, K.M.; Ward, S.M. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J. Physiol. 2001, 537, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Mazzia, C.; Porcher, C.; Jule, Y.; Christen, M.O.; Henry, M. Ultrastructural study of relationships between c-kit immunoreactive interstitial cells and other cellular elements in the human colon. Histochem. Cell Biol. 2000, 113, 401–411. [Google Scholar] [PubMed]
- Beckett, E.A.; Ro, S.; Bayguinov, Y.; Sanders, K.M.; Ward, S.M. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev. Dyn. 2007, 236, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Torihashi, S.; Ward, S.M.; Nishikawa, S.; Nishi, K.; Kobayashi, S.; Sanders, K.M. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995, 280, 97–111. [Google Scholar] [PubMed]
- Chang, I.Y.; Glasgow, N.J.; Takayama, I.; Horiguchi, K.; Sanders, K.M.; Ward, S.M. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J. Physiol. 2001, 536, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Yanase, H.; Sanders, K.M.; Ward, S.M. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology 2004, 127, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Vannucchi, M.G.; Nieuwmeyer, F.; Ye, J.; Faussone-Pellegrini, M.S.; Huizinga, J.D. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am. J. Pathol. 2005, 167, 437–453. [Google Scholar] [CrossRef]
- Mei, F.; Zhu, J.; Guo, S.; Zhou, D.S.; Han, J.; Yu, B.; Li, S.F.; Jiang, Z.Y.; Xiong, C.J. An age-dependent proliferation is involved in the postnatal development of interstitial cells of Cajal in the small intestine of mice. Histochem. Cell Biol. 2009, 131, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Stanich, J.E.; Gibbons, S.J.; Eisenman, S.T.; Bardsley, M.R.; Rock, J.R.; Harfe, B.D.; Ordog, T.; Farrugia, G. Ano1 as a regulator of proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1044–G1051. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Gibbons, S.J.; Roeder, J.L.; Lurken, M.S.; Zhu, J.; Wouters, M.M.; Miller, S.M.; Szurszewski, J.H.; Farrugia, G. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol. Motil. 2007, 19, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Gibbons, S.J.; Roeder, J.L.; Distad, M.; Ou, Y.; Strege, P.R.; Szurszewski, J.H.; Farrugia, G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology 2007, 133, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.W.; Parsons, S.P.; Huizinga, J.D. Ca2+ sensitivity of the maxi chloride channel in interstitial cells of Cajal. Neurogastroenterol. Motil. 2012, 24, e221–e234. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.P.; Huizinga, J.D. Transient outward potassium current in ICC. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G456–G466. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.J.; Hwang, S.J.; Bayguinov, Y.; Colletti, E.J.; Sanders, K.M.; Ward, S.M. Establishment of pacemaker activity in tissues allotransplanted with interstitial cells of Cajal. Neurogastroenterol. Motil. 2013, 25, e418–e428. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yoshikawa, M.; Takaki, M.; Torihashi, S.; Kato, Y.; Nakajima, Y.; Ishizaka, S.; Tsunoda, Y. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells 2002, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Ogaeri, T.; Matsuura, R.; Kogo, H.; Fujimoto, T.; Torihashi, S. In vitro organogenesis of gut-like structures from mouse embryonic stem cells. Neurogastroenterol. Motil. 2004, 16, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Yamada, T.; Hokuto, D.; Koyama, F.; Kasuda, S.; Kanehiro, H.; Nakajima, Y. Generation of functional gut-like organ from mouse induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2010, 391, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.L.; Mahe, M.M.; Munera, J.; Howell, J.C.; Sundaram, N.; Poling, H.M.; Schweitzer, J.I.; Vallance, J.E.; Mayhew, C.N.; Sun, Y.; et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014, 20, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Thuneberg, L.; Kluppel, M.; Malysz, J.; Mikkelsen, H.B.; Bernstein, A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shamu, T.; Chen, H.; Besmer, P.; Sawyers, C.L.; Chi, P. Visualization of the interstitial cells of cajal (ICC) network in mice. J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, P.J.; Gibbons, S.J.; Bardsley, M.R.; Lorincz, A.; Pozo, M.J.; Pasricha, P.J.; Van de Rijn, M.; West, R.B.; Sarr, M.G.; Kendrick, M.L.; et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1370–G1381. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Wang, X.Y.; Wright, G.W.; Barajas-Lopez, C.; Huizinga, J.D. Ano1 is a better marker than c-Kit for transcript analysis of single interstitial cells of Cajal in culture. Cell. Mol. Biol. Lett. 2014, 19, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, J.H.; Li, K.L.; Zhu, Y.F.; Wright, G.W.J.; Huizinga, J.D. Discrepancies between c-Kit positive and Ano1 positive ICC-SMP in the W/W-v and wild-type mouse colon; relationships with motor patterns and calcium transients. Neurogastroenterol. Motil. 2014, 26, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Visel, A.; Thaller, C.; Eichele, G. GenePaint.org: An atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004, 32, D552–D556. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.; Bernard, C.E.; Strege, P.R.; Beyder, A.; Galietta, L.J.; Pasricha, P.J.; Rae, J.L.; Parkman, H.P.; Linden, D.R.; Szurszewski, J.H.; et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J. Biol. Chem. 2011, 286, 13393–13403. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Godfrey, T.E.; Gooding, W.E.; McCarty, K.S., Jr.; Gollin, S.M. Comprehensive genome and transcriptome analysis of the 11q13 amplicon in human oral cancer and synteny to the 7F5 amplicon in murine oral carcinoma. Genes Chromosomes Cancer 2006, 45, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Blair, P.J.; Britton, F.C.; O’Driscoll, K.E.; Hennig, G.; Bayguinov, Y.R.; Rock, J.R.; Harfe, B.D.; Sanders, K.M.; Ward, S.M. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 2009, 587, 4887–4904. [Google Scholar] [CrossRef] [PubMed]
- Lavin, S.T.; Southwell, B.R.; Murphy, R.; Jenkinson, K.M.; Furness, J.B. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem. Cell Biol. 1998, 110, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Portbury, A.L.; Furness, J.B.; Young, H.M.; Southwell, B.R.; Vigna, S.R. Localisation of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. J. Comp. Neurol. 1996, 367, 342–351. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Faussone-Pellegrini, M.S. NK1, NK2 and NK3 tachykinin receptor localization and tachykinin distribution in the ileum of the mouse. Anat. Embryol. 2000, 202, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Wong, H.; Wu, S.V.; de Giorgio, R.; Yang, M.; Reeve, J., Jr.; Brecha, N.C.; Walsh, J.H. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J. Comp. Neurol. 1997, 386, 396–408. [Google Scholar] [CrossRef]
- Burnstock, G.; Lavin, S. Interstitial cells of Cajal and purinergic signalling. Auton. Neurosci. 2002, 97, 68–72. [Google Scholar] [CrossRef]
- Poole, D.P.; Van Nguyen, T.; Kawai, M.; Furness, J.B. Protein kinases expressed by interstitial cells of Cajal. Histochem. Cell Biol. 2004, 121, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Southwell, B.R. Localization of protein kinase C theta immunoreactivity to interstitial cells of Cajal in guinea-pig gastrointestinal tract. Neurogastroenterol. Motil. 2003, 15, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, C.W.; Xue, C.; Ward, S.M.; de Vente, J.; Sanders, K.M. Immunohistochemical localization of 3′,5′-cyclic guanosine monophosphate in the canine proximal colon: Responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience 1993, 56, 513–522. [Google Scholar] [CrossRef]
- Young, H.M.; McConalogue, K.; Furness, J.B.; De Vente, J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience 1993, 55, 583–596. [Google Scholar] [CrossRef]
- Koh, S.D.; Sanders, K.M.; Ward, S.M. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J. Physiol. 1998, 513, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hatton, W.J.; Mason, H.S.; Carl, A.; Doherty, P.; Latten, M.J.; Kenyon, J.L.; Sanders, K.M.; Horowitz, B. Functional and molecular expression of a voltage-dependent K(+) channel (Kv1.1) in interstitial cells of Cajal. J. Physiol. 2001, 533, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.; Robinson, T.L.; Lee, J.C.; Farraway, L.A.; Hughes, M.J.; Andrews, D.W.; Huizinga, J.D. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat. Med. 1998, 4, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Ordog, T.; Redelman, D.; Miller, L.J.; Horvath, V.J.; Zhong, Q.; Almeida-Porada, G.; Zanjani, E.D.; Horowitz, B.; Sanders, K.M. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am. J. Physiol. Cell Physiol. 2004, 286, C448–C456. [Google Scholar] [CrossRef] [PubMed]
- Ordog, T.; Redelman, D.; Horowitz, N.N.; Sanders, K.M. Immunomagnetic enrichment of interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G351–G360. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Redelman, D.; Ro, S.; Ward, S.M.; Ordog, T.; Sanders, K.M. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am. J. Physiol. Cell Physiol. 2007, 292, C497–C507. [Google Scholar] [CrossRef] [PubMed]
- Beckett, E.A.; Bayguinov, Y.R.; Sanders, K.M.; Ward, S.M.; Hirst, G.D. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J. Physiol. 2004, 559, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Ordog, T.; Koh, S.D.; Baker, S.A.; Jun, J.Y.; Amberg, G.; Monaghan, K.; Sanders, K.M. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J. Physiol. 2000, 525, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ordog, T.; Baldo, M.; Danko, R.; Sanders, K.M. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/W mutant mice. Gastroenterology 2002, 123, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ordog, T.; Koh, S.D.; Ward, S.M. A Novel Pacemaker Mechanism Drives Gastrointestinal Rhythmicity. News Physiol. Sci. 2000, 15, 291–298. [Google Scholar] [PubMed]
- Liu, L.W.; Thuneberg, L.; Huizinga, J.D. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am. J. Physiol. 1994, 266, G485–G496. [Google Scholar] [PubMed]
- Torihashi, S.; Nishi, K.; Tokutomi, Y.; Nishi, T.; Ward, S.; Sanders, K.M. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology 1999, 117, 140–148. [Google Scholar] [CrossRef]
- Matsuura, T.; Masumoto, K.; Ieiri, S.; Nakatsuji, T.; Akiyoshi, J.; Nishimoto, Y.; Takahashi, Y.; Hayashida, M.; Taguchi, T. Morphological and physiological changes of interstitial cells of Cajal after small bowel transplantation in rats. Transpl. Int. 2007, 20, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Won, K.J.; Suzuki, T.; Hori, M.; Ozaki, H. Motility disorder in experimentally obstructed intestine: Relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol. Motil. 2006, 18, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Camborova, P.; Hubka, P.; Sulkova, I.; Hulin, I. The pacemaker activity of interstitial cells of Cajal and gastric electrical activity. Physiol. Res. 2003, 52, 275–284. [Google Scholar] [PubMed]
- Sandgren, K.; Ekblad, E.; Larsson, L.T. Survival of neurons and interstitial cells of Cajal after autotransplantation of myenteric ganglia from small intestine in the lethal spotted mouse. Pediatr. Surg. Int. 2000, 16, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yamagata, A.; Nishikawa, S.; Yoshinaga, K.; Kobayashi, S.; Nishi, K.; Nishikawa, S. Requirement of c-kit for development of intestinal pacemaker system. Development 1992, 116, 369–375. [Google Scholar] [PubMed]
- Chabot, B.; Stephenson, D.A.; Chapman, V.M.; Besmer, P.; Bernstein, A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 1988, 335, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Burns, A.J.; Torihashi, S.; Sanders, K.M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 1994, 480, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Nakamura, T.; Sato, T.; Nemoto, Y.; Mizutani, T.; Zheng, X.; Ichinose, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat. Med. 2012, 18, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Lindley, R.M.; Hawcutt, D.B.; Connell, M.G.; Almond, S.L.; Vannucchi, M.G.; Faussone-Pellegrini, M.S.; Edgar, D.H.; Kenny, S.E. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology 2008, 135, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M. Interstitial cells of Cajal in enteric neurotransmission. Gut 2000, 47, iv40–iv43. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, F.; Steinbeck, J.A.; Kriks, S.; Tchieu, J.; Zimmer, B.; Kishinevsky, S.; Zeltner, N.; Mica, Y.; El-Nachef, W.; Zhao, H.; et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 2016, 531, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, L.; Zeng, J.; Lin, W.; Li, K.; Sun, J.; Huang, W.; Chen, J.; Wang, G.; Ke, Q.; et al. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol. Psychiatry 2016. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, E.; Elbahrawy, M.; Orlando, G.; Bitar, K.N. Successful implantation of an engineered tubular neuromuscular tissue composed of human cells and chitosan scaffold. Surgery 2015, 158, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, E.; Raghavan, S.; Bitar, K.N. Neo-innervation of a bioengineered intestinal smooth muscle construct around chitosan scaffold. Biomaterials 2014, 35, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Rego, S.L.; Shannon, B.; Zakhem, E.; Bitar, K.N. Incorporation of interstitial cells of Cajal in bioengineered interinsically innervated smooth muscle constructs. Gastroenterology 2015, 148. [Google Scholar] [CrossRef]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Speer, A.L.; Sala, F.G.; Matthews, J.A.; Grikscheit, T.C. Murine tissue-engineered stomach demonstrates epithelial differentiation. J. Surg. Res. 2011, 171, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E.; Barthel, E.R.; Speer, A.L.; Sala, F.G.; Hou, X.; Torashima, Y.; Grikscheit, T.C. Human tissue-engineered small intestine forms from postnatal progenitor cells. J. Pediatr. Surg. 2013, 48, 129–137. [Google Scholar] [CrossRef] [PubMed]

| Subtypes | Localisation | Morphology | Functions | Differential Markers | References |
|---|---|---|---|---|---|
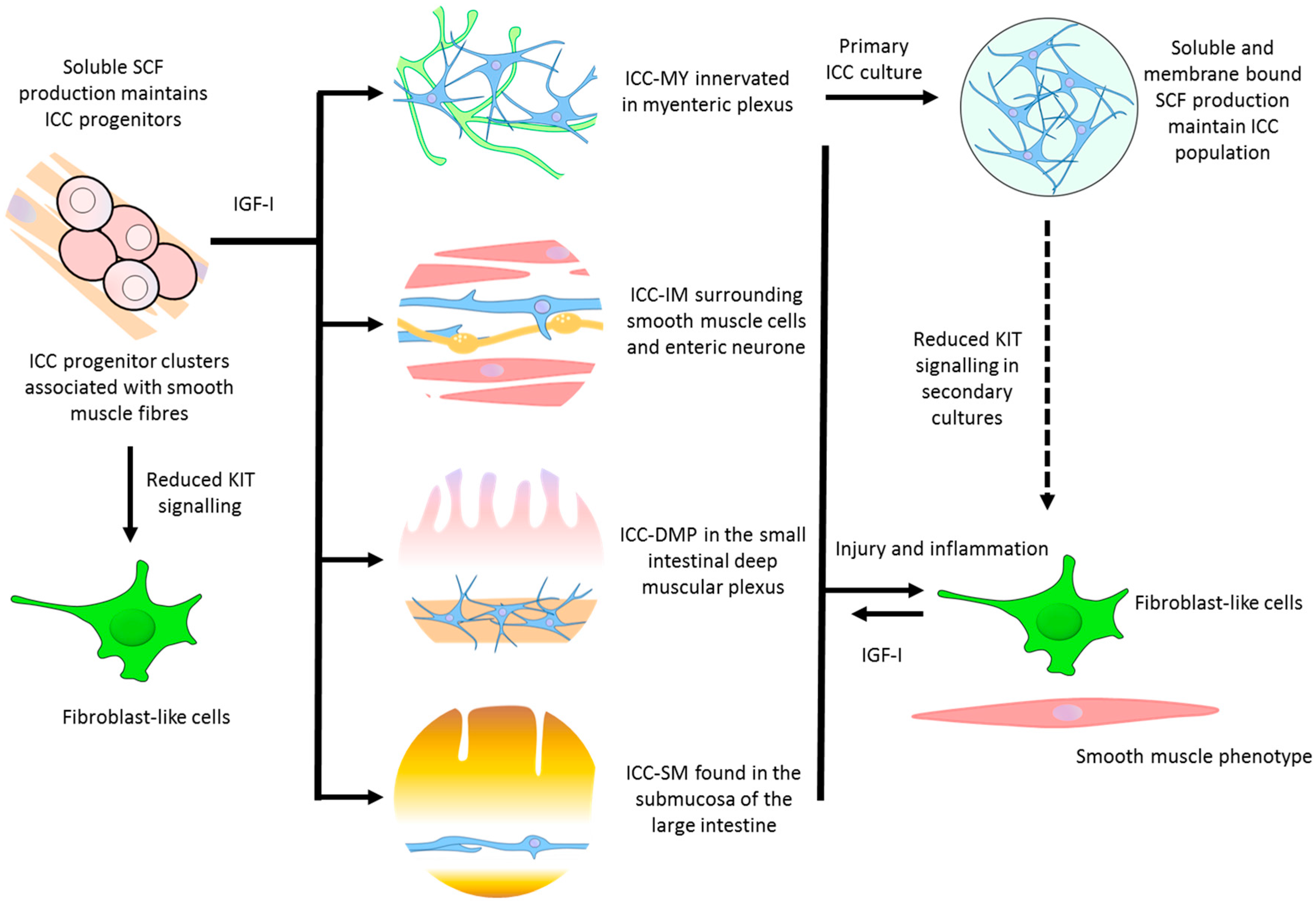
| ICC progenitor | - Stomach - Intestine | Densely packed clusters of oval or circular cells extending within the tunica muscularis | Precursor ICC that can replenish damaged or lost ICC. Smooth muscle produced soluble stem cell factor (SCF) is responsible for its partial commitment into mature ICC | Kitlow, CD44, CD34, InsR, IGF-IR | [30] |
| ICC-MY | - Stomach (antrum only) - Small intestine - Large intestine | Multipolar cells with branched processes connecting to each other and forming a network around the myenteric plexus in the space between circular and longitudinal muscle layers | - The dominant pacemakers in gastric muscle that generate slow-waves activity - ICC-smooth muscle coupling; electronically coupled via gap junctions or direct contact to propagate slow-waves from ICC to smooth muscle | Kit, Ano1, M2, M3, VIP-1, SCF-A, NK3 | [9,12,31] |
| ICC-IM | - Distal oesophagus - Stomach - Pylorus - Small intestine - Large intestine | Bipolar or spindle-shaped cells associated with circular muscle (ICC-CM) or longitudinal (ICC-LM) muscle. Also occur in the connective tissue septa (ICC-SEP) | - Produce spontaneous depolarisations (unitary potentials) that generate low-frequency slow-waves - Mediate neural transmission between enteric nerves and smooth muscle - Stretch sensitivity in gastric muscles | Kit, Ano1, M2, M3, VIP-1, SCF-A, NK1, NK3 | [15,31,32,33] |
| ICC-DMP | - Small intestine | Multipolar cells associated with the nerve bundles of the deep muscular plexus | - Mediate neural transmission in small intestine | Kit, Ano1, NK1, NK3 | [15,34] |
| Others | - Pylorus (ICC-SM) - Large intestine (ICC-SMP) | Bipolar and multipolar ICC found in the submucosa (ICC-SM) and submucosal plexus (ICC-SMP) lie between the submucosal connective tissue and the innermost circular muscle layer | - Neurotransmission and pacemaker roles | Kit, Ano1, | [35,36] |
| Cultured ICC | - Cultures from freshly dissociated tissue | Primary culture consists of extensive network of multipolar ICC on the surface of smooth muscle cells. Secondary cultures contain a mix of multipolar and bipolar ICC. Networks of fibroblast-like cells and smooth muscle cells appear. | - Pacemaker properties generating contractility - A change towards a smooth muscle phenotype | Kit, Ano1, Smooth muscle myosin, M2, M3, VIP-1, VIP-2, SCF-A, SCF-B | [31] |
| Technique | Tissue/Total Cell Count | ICC Isolated | Reference |
|---|---|---|---|
| Visual identification | Mouse small intestine | 38–38 | [78] |
| Kit labelled FACS | Mouse small intestine 1.2–2.8 × 106 cells | 30,000–40,000 | [79] |
| Immunomagnetic depletion of macrophages and Kit labelled FACS | Mouse small intestine 1.2–2.8 × 106 cells | 2000–4000 | [79] |
| Kit labelled MACS | Mouse small intestine 0.86–1.12 × 106 cells | 7100 | [80] |
| Kit and substance P labelled FACS | Mouse small intestine 15 × 1.5 mm strips | 12,000 ICC-DMP/ICC-IM, 55,000 ICC-MY | [81] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; O’Connor, M.D.; Ho, V. The Potential for Gut Organoid Derived Interstitial Cells of Cajal in Replacement Therapy. Int. J. Mol. Sci. 2017, 18, 2059. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102059
Zhou J, O’Connor MD, Ho V. The Potential for Gut Organoid Derived Interstitial Cells of Cajal in Replacement Therapy. International Journal of Molecular Sciences. 2017; 18(10):2059. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102059
Chicago/Turabian StyleZhou, Jerry, Michael D. O’Connor, and Vincent Ho. 2017. "The Potential for Gut Organoid Derived Interstitial Cells of Cajal in Replacement Therapy" International Journal of Molecular Sciences 18, no. 10: 2059. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102059