H2O2 Is Involved in the Metallothionein-Mediated Rice Tolerance to Copper and Cadmium Toxicity
Abstract
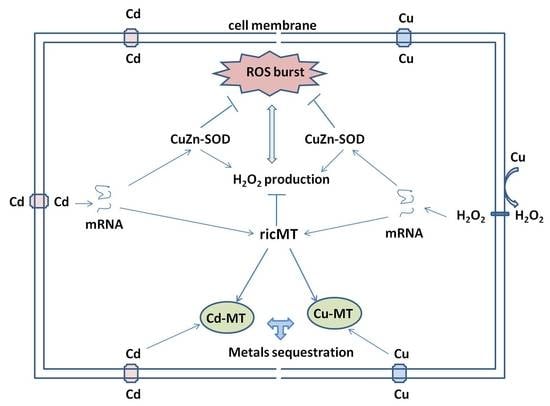
:1. Introduction
2. Results
2.1. Effects of Cu and Cd on H2O2 Production in Rice Radicles
2.2. Cu and Cd Up-Regulate the ricMT and CuZn-SOD Gene Expression in Rice Radicles
2.3. ricMT Expression Improved Cu and Cd Tolerance of Rice Suspension Cells
2.4. ricMT Expression Decreased H2O2 Production in Rice Suspension Cells under Cu and Cd Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Generation of Sense and Antisense ricMT Transgenic Rice
4.3. Suspension Cell Cultures
4.4. Total RNA Isolation, cDNA Synthesis and Quantitative RT-PCR
4.5. Hydrogen Peroxide Localization In Situ
4.6. H2O2 Determination in Extracts
4.7. Evans Blue Assay for Suspension Cell Death
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Abbreviations
| Cys | cysteine |
| ROS | reactive oxygen species |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| MT | metallothionein |
| SOD | superoxide dismutase |
| WT | wild-type |
References
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Ann. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Cho, U.H.; Seo, N.H. Oxidative stress in arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci. 2005, 168, 113–120. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Hsu, Y.T.; Kao, C.H. Involvement of glutathione in heat shock- and hydrogen peroxide-induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 2009, 318, 37–45. [Google Scholar] [CrossRef]
- Hu, Y.; Ge, Y.; Zhang, C.; Ju, T.; Cheng, W. Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul. 2009, 59, 51–61. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Shyam Sunder, A.; Manisenthil Kumar, K.T.; Senthil kumar, M.; Ganesh, G.N.K.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Mir, G.; Domenech, J.; Huguet, G.; Guo, W.J.; Goldsbrough, P.; Atrian, S.; Molinas, M. A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. J. Exp. Bot. 2004, 55, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kushwaha, H.R.; Panjabi-Sabharwal, V.; Kumari, S.; Joshi, R.; Karan, R.; Mittal, S.; Pareek, S.L.S.; Pareek, A. Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol. 2012, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, Y.; Li, Y.; Ling, H.Q.; Chu, C. Osmt1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol. Biol. 2009, 70, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, D.X.; Miao, Q.; Xue, T.T.; Li, X.Z.; Zheng, C.C. Arabidopsis thaliana metallothionein, AtMT2a, mediates ROS balance during oxidative stress. J. Plant Biol. 2009, 52, 585–592. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Song, W.Y.; Choi, K.S.; Hur, Y. Chloroplast-targeted BrMT1 (Brassica rapa type-1 metallothionein) enhances resistance to cadmium and ROS in transgenic Arabidopsis plants. J. Plant Biol. 2007, 50, 1–7. [Google Scholar] [CrossRef]
- Xia, Y.; Qi, Y.; Yuan, Y.; Wang, G.; Cui, J.; Chen, Y.; Zhang, H.; Shen, Z. Overexpression of Elsholtzia haichowensis metallothionein 1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. J. Hazard. Mater. 2012, 233, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Samardzic, J.T.; Nikolic, D.B.; Timotijevic, G.S.; Jovanovic, Z.S.; Milisavljevic, M.D.; Maksimovic, V.R. Tissue expression analysis of FeMT3, a drought and oxidative stress related metallothionein gene from buckwheat (Fagopyrum esculentum). J. Plant Physiol. 2010, 167, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Tian, S.; Lu, L.; Shohag, M.J.I.; Yang, X. Metallothionein 2 (SaMT2) from Sedum alfredii hance confers increased Cd tolerance and accumulation in yeast and tobacco. PLoS ONE 2014, 9, e102750. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, X.; Qian, M.; Zheng, L.; Lian, C.; Xia, Y.; Shen, Z. Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J. Hazard. Mater. 2015, 294, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Deng, X.; Quan, L.; Xia, Y.; Shen, Z. Metallothioneins BcMT1 and BcMT2 from Brassica campestris enhance tolerance to cadmium and copper and decrease production of reactive oxygen species in Arabidopsis thaliana. Plant Soil 2012, 367, 507–519. [Google Scholar] [CrossRef]
- Wong, H.L.; Sakamoto, T.; Kawasaki, T.; Umemura, K.; Shimamoto, K. Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004, 135, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xu, Y.; Li, J.; Yang, L.; Liu, J.Y. Molecular analyses of the metallothionein gene family in rice (Oryza sativa L.). J. Biochem. Mol. Biol. 2006, 39, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Sauter, M. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 2009, 21, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Umeda, M.; Liu, J.Y.; Zhao, N.M.; Uchimiya, H. A novel MT gene of rice plants is strongly expressed in the node portion of the stem. Gene 1998, 206, 29–35. [Google Scholar] [CrossRef]
- Zhang, H.; Lian, C.; Shen, Z. Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Ann. Bot. 2009, 103, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, Y.; Zhuang, K.; Li, L.; Xia, Y.; Shen, Z. Proteomic analysis of copper-binding proteins in excess copper-stressed roots of two rice (Oryza sativa L.) varieties with different Cu tolerances. PLoS ONE 2015, 10, e0125367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Xia, Y.; Chen, C.; Zhuang, K.; Song, Y.F.; Shen, Z.G. Analysis of copper-binding proteins in rice radicles exposed to excess copper and hydrogen peroxide stress. Front. Plant Sci. 2016, 7, 1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper-zinc superoxide dismutase in roots of Elsholtzia haichowensis. Planta 2008, 227, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces production of hydrogen peroxide in the leaf of Elsholtzia haichowensis through apoplastic and symplastic CuZn-superoxide dismutase. J. Hazard. Mater. 2010, 178, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xia, Y.; Hu, W.; Zhang, H.; Shen, Z. Cadmium-induced oxidative damage and protective effects of N-acetyl-l-cysteine against cadmium toxicity in Solanum nigrum L. J. Hazard. Mater. 2010, 180, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, H.; Xia, Y.; Wang, G.; Xu, L.; Shen, Z. Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep. 2011, 30, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Cohu, C.M.; Abdel-Ghany, S.E.; Reynolds, K.A.G.; Onofrio, A.M.; Bodecker, J.R.; Kimbrel, J.A.; Niyogi, K.K.; Pilon, M. Copper delivery by the copper chaperone for chloroplast and cytosolic copper/zinc-superoxide dismutases: Regulation and unexpected phenotypes in an Arabidopsis mutant. Mol. Plant 2009, 2, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, S.; van der Kelen, K.; Dat, J.; Gadjev, I.; Boonefaes, T.; Morsa, S.; Rottiers, P.; Slooten, L.; van Montagu, M.; Zabeau, M.; et al. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc. Natl. Acad. Sci. USA 2003, 100, 16113–16118. [Google Scholar] [CrossRef] [PubMed]
- Yoon-Sik, K.; Hyun-Soon, K.; Yong-Hwa, L.; Mi-Sun, K.; Hyun-Woo, O.; Kyu-Woong, H.; Hyouk, J.; Jae-Heung, J. Elevated H2O2 production via overexpression of a chloroplastic Cu/Zn SOD gene of lily (Lilium oriental hybrid ‘marco polo’) triggers ethylene synthesis in transgenic potato. Plant Cell Rep. 2008, 27, 973–983. [Google Scholar]
- Carri, M.T.; Galiazzo, F.; Ciriolo, M.R.; Rotilio, G. Evidence for co-regulation of Cu,Zn superoxide dismutase and metallothionein gene expression in yeast through transcriptional control by copper via the ACE1 factor. FEBS Lett. 1991, 278, 263–266. [Google Scholar] [CrossRef]
- Taylor, D.M.; Minotti, S.; Agar, J.N.; Durham, H.D. Overexpression of metallothionein protects cultured motor neurons against oxidative stress, but not mutant Cu/Zn-superoxide dismutase toxicity. Neurotoxicology 2004, 25, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, P.; Chen, H.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.T.; Jiang, L.; Wu, K.; Huang, S. Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in arabidopsis. Planta 2011, 235, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yao, W.; Wang, S.; Wang, X.; Jiang, T. The metallothionein gene, TaMT3, from Tamarix androssowii confers Cd2+ tolerance in tobacco. Int. J. Mol. Sci. 2014, 15, 10398–10409. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, R.M.; Shahpiri, A.; Mirlohi, A. Discrimination between two rice metallothionein isoforms belonging to type 1 and type 4 in metal-binding ability. Biotechnol. Appl. Biochem. 2013, 60, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Li, X.; Zhu, W.; Wu, C.; Yang, G.; Zheng, C. Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J. Exp. Bot. 2009, 60, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Liu, G.; Yang, C.; Li, C. Tamarix hispida metallothionein-like ThMT3, a reactive oxygen species scavenger, increases tolerance against Cd2+, Zn2+, Cu2+, and NaCl in transgenic yeast. Mol. Biol. Rep. 2010, 38, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Dayananda, S.; Subramanyam, C. Copper alone, but not oxidative stress, induces copper-metallothionein gene in Neurospora crassa. FEMS Microbiol. lett. 2005, 242, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Aichi, I.; Matsuoka, M. A protocol for Agrobacterium-mediated transformation in rice. Nat. Protoc. 2007, 1, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, B.; Gardner, R.C.; Ezaki, Y.; Matsumoto, H. Expression of aluminum-induced genes in transgenic arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 2000, 122, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Zhang, J.H. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Mock, N.M. An improved method for monitoring cell death in cellsuspension and leaf disc assays using evans blue. Plant Cell Tiss. Org. 1994, 39, 7–12. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lv, S.; Xu, H.; Hou, D.; Li, Y.; Wang, F. H2O2 Is Involved in the Metallothionein-Mediated Rice Tolerance to Copper and Cadmium Toxicity. Int. J. Mol. Sci. 2017, 18, 2083. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102083
Zhang H, Lv S, Xu H, Hou D, Li Y, Wang F. H2O2 Is Involved in the Metallothionein-Mediated Rice Tolerance to Copper and Cadmium Toxicity. International Journal of Molecular Sciences. 2017; 18(10):2083. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102083
Chicago/Turabian StyleZhang, Hongxiao, Shufang Lv, Huawei Xu, Dianyun Hou, Youjun Li, and Fayuan Wang. 2017. "H2O2 Is Involved in the Metallothionein-Mediated Rice Tolerance to Copper and Cadmium Toxicity" International Journal of Molecular Sciences 18, no. 10: 2083. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102083