Detection and Management of Mango Dieback Disease in the United Arab Emirates
Abstract
:1. Introduction
2. Results
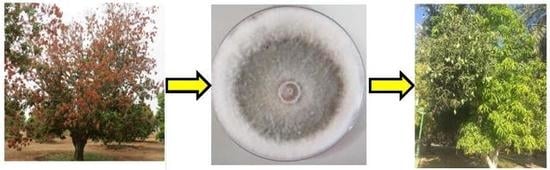
2.1. Symptoms of Dieback Disease on Mango
2.2. Morphological and Phylogenetic Identification of L. theobromae Associated with Dieback Disease
2.3. Pathogenicity Tests of L. theobromae on Mango Leaves, Fruits, and Seedlings
2.4. In Vitro Evaluation of Systemic Fungicides Against L. theobromae
2.5. Effect of Fungicides on Mango Plants Infected with L. theobromae
3. Discussion
4. Materials and Methods
4.1. Fungal Isolation and Purification
4.2. DNA Isolation, PCR, and Sequencing
4.3. Phylogenetic Analysis
4.4. Disease Assays and Pathogenicity Tests
4.5. Evaluation of Fungicides Against L. theobromae
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O- and xanthone C-glycosides, anthocyanins, and pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S. Mango anthracnose (Colletotrichum gloeosporioides). Plant Dis. 2008, 48, 1–9. [Google Scholar]
- Prakash, O. Compendium of Mango Diseases and Disorders; Vedams eBooks (P) Ltd.: New Delhi, India, 2003; p. 84. [Google Scholar]
- Ploetz, R.C. Diseases of mango. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; APS Press: St. Paul, MN, USA, 2003; pp. 327–363. [Google Scholar]
- Ploetz, R.C. The major diseases of mango: Strategies and potential for sustainable management. Acta Hortic. 2004, 645, 137–150. [Google Scholar] [CrossRef]
- Smith, P.F.; Scudder, G.K. Some studies of mineral deficiency symptoms in mango. Proc. Florida State Hort. Soc. 1951, 64, 243–248. [Google Scholar]
- Ramos, L.J.; Lara, S.P.; McMillan, R.T.; Narayanan, K.R. Tip die back of mango (Mangifera indica) caused by Botryosphaeria ribis. Plant Dis. 1991, 75, 315–318. [Google Scholar] [CrossRef]
- Slippers, B.; Johnson, G.I.; Crous, P.W.; Coutinho, T.A.; Wingfield, B.; Wingfield, M.J. Phylogenetic and morphological re-evolution of the Botryosphaeria species causing diseases of Mangifera indica. Mycologia 2005, 97, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, M.; Al-Adawi, A.O.; Wingfield, B.D.; Al-Subhi, A.M.; Deadman, M.L.; Wingfield, M.J. DNA based characterization of Ceratocystis fimbriata isolates associated with mango decline in Oman. Australas. Plant Pathol. 2005, 34, 587–590. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E.ST.; Burgess, T.I. Pathogenic Botryosphaeriacea associated with Mangifera indica in the Kimberley region of Western Australia. Eur. J. Plant Pathol. 2011, 130, 379–391. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Mohammadi, G.E.; Zare, R.; Phillips, A.J.L. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Cirvilleri, G.; Polizzi, G.; Crous, P.W.; Groenewald, J.Z.; Lombard, L. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas. Plant Pathol. 2012, 41, 649–660. [Google Scholar] [CrossRef]
- Zambettakis, E.C. Recherches sur la systematique des “Sphaeropsidales-Phaeodidymae”. Bull. Trimest. Soc. Mycol. Fr. 1954, 70, 219–349. [Google Scholar]
- Sutton, B.C. The Coelomycetes, Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Surrey, UK, 1980. [Google Scholar]
- Sharma, I.M.; Raj, H.; Kaul, J.L. Studies on postharvest diseases of mango and chemical control of stem end rot and anthracnose. Indian Phytopathol. 1994, 47, 197–200. [Google Scholar]
- Ploetz, R.C.; Benscher, D.; Vázquez, A.; Colls, A.; Nagel, J.; Schaffer, B. A re-examination of mango decline in Florida. Plant Dis. 1996, 80, 664–668. [Google Scholar] [CrossRef]
- Al Adawi, A.O.; Deadman, M.L.; Al Rawahi, A.K.; Khan, A.J.; Al Maqbali, Y.M. Diplodia theobromae associated with sudden decline of mango in the Sultanate of Oman. Plant Pathol. 2003, 52, 419. [Google Scholar] [CrossRef]
- Khanzada, M.A.; Lodhi, A.M.; Shahzad, S. Chemical control of Lasiodiplodia theobromae, the causal agent of mango decline in Sindh. Pak. J. Bot. 2005, 37, 1023–1030. [Google Scholar]
- De Oliveira Costa, V.S.; Michereff, S.J.; Martins, R.B.; Gava, C.A.T.; Mizubuti, E.S.G.; Câmara, M.P.S. Species of Botryosphaeriaceae associated on mango in Brazil. Eur. J. Plant Pathol. 2010, 127, 509–519. [Google Scholar] [CrossRef]
- Hong, S.K.; Lee, S.Y.; Choi, H.W.; Lee, Y.K.; Joa, J.H.; Shim, H. Occurrence of stem-end rot on mango fruits caused by Lasiodiplodia theobromae in Korea. Plant Pathol. J. 2012, 28, 455. [Google Scholar] [CrossRef]
- Haggag, W.M. Mango diseases in Egypt. Agric. Biol. J. N. Am. 2010, 1, 285–289. [Google Scholar] [CrossRef]
- Khanzada, M.A.; Lodhi, A.M.; Shahzad, S. Mango dieback and gummosis in Sindh, Pakistan caused by Lasiodiplodia theobromae. Plant Health Prog. 2004. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Perveen, R.; Malik, M.T.; Malik, O.; Umer, U.D.; Wazeer, M.S.; Rehman, A.; Majid, T.; Abbas, Z. Characterization of symptoms severity on various mango cultivars to quick decline of mango in district Multan. Int. J. Biosci. 2014, 4, 157–163. [Google Scholar]
- Khanzada, M.A.; Lodhi, A.M.; Rajput, A.Q.; Syed, R.N.; Shahzad, S. Response of different mango cultivars to mango decline pathogen, Lasiodiplodia theobromae Pat. Int. J. Biol. Biotechnol. 2015, 12, 643–647. [Google Scholar]
- Naqvi, S.A.H.; Perveen, R. Mango quick decline manifestation on various cultivars at plants of particular age in the vicinity of district Multan. Pak. J. Phytopathol. 2015, 27, 31–39. [Google Scholar]
- Kazmi, M.; Fateh, F.; Majeed, K.; Kashkhely, A.M.; Hussain, I.; Ahmad, I.; Jabeen, A. Incidence and etiology of mango sudden death phenomenon in Pakistan. Pak. J. Phytopathol. 2005, 17, 154–158. [Google Scholar]
- Paolinelli-Alfonso, M.; Villalobos-Escobedo, J.M.; Rolshausen, P.; Herrera-Estrella, A.; Galindo-Sánchez, C.; López-Hernández, J.F.; Hernandez-Martinez, R. Global transcriptional analysis suggests Lasiodiplodia theobromae pathogenicity factors involved in modulation of grapevine defensive response. BMC Genom. 2016, 17, 615. [Google Scholar] [CrossRef] [PubMed]
- Alemu, K. Dynamics and management of major postharvest fungal diseases of mango fruits. J. Biol. Agric. Healthc. 2014, 4, 13–21. [Google Scholar]
- Asrey, R.; Patel, V.B.; Barman, K.; Pal, R.K. Pruning affects fruit yield and postharvest quality in mango (Mangifera indica L.) cv. Amrapali. Fruits 2013, 68, 367–380. [Google Scholar] [CrossRef]
- Garg, N.; Pathak, O.; Pathak, R.K. Use of Cow Dung Paste for Controlling Gummosis and Die Back Diseases of Mango. In Proceedings of the 43rd Annual Conference of Association of Microbiologists of India, Hisar (Haryana), India, 11–13 December 2002; p. 171. [Google Scholar]
- Gupta, V.P.; Tewar, S.K.; Govidaiah; Bajpai, A.K. Ultrastructure of mycoparasitisms of Trichoderma, Gliocladium and Laetisaria species on Botryodiplodia theobromae. J. Phytopathol. 1999, 147, 19–24. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Rao, M.S. Evaluation of Trichoderma viride antagonistic to post harvest pathogens on mango. Indian Phytopathol. 2001, 54, 493–494. [Google Scholar]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Fatima, B.; Muhammad, M.J. Breeding in Mango. Int. J. Agric. Biol. 2001, 3, 522–526. [Google Scholar]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted health benefits of Mangifera indica L. (Mango): The inestimable value of orchards recently planted in sicilian rural. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Punithalingam, E. Plant Diseases Attributed to Botryodiplodia theobromae Pat; J. Cramer: Vaduz, Liechtenstein, 1980; p. 121. [Google Scholar]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridization in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gálvez, E.; Alves, A. Identification and pathogenicity of Lasiodiplodia theobromae causing dieback of table grapes in Peru. Eur. J. Plant Pathol. 2015, 141, 477–489. [Google Scholar] [CrossRef]
- Mbenoun, M.; Momo Zeutsa, E.H.; Samuels, G.; Nsouga Amougou, F.; Nyasse, S. Dieback due to Lasiodiplodia theobromae, a new constraint to cocoa production in Cameroon. New Dis. Rep. 2007, 15, 59. [Google Scholar] [CrossRef]
- Amusa, N.A.; Adegbite, A.A.; Muhammed, S.; Baiyewu, R.A. Yam disease and its management in Nigeria. Afr. J. Biotechnol. 2003, 2, 497–502. [Google Scholar] [CrossRef]
- Twumasi, P.; Ohene-Mensah, G.; Moses, E. The rot fungus Botryodiplodia theobromae strains cross infect cocoa, mango, banana and yam with significant tissue damage and economic losses. Afr. J. Agric. Res. 2014, 9, 613–619. [Google Scholar]
- Rodríguez-Gálvez, E.; Guerrero, P.; Barradas, C.; Crous, P.W.; Alves, A. Phylogeny and pathogenicity of Lasiodiplodia species associated with dieback of mango in Peru. Fungal Biol. 2017, 121, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, D.; Slippers, B.; Coutinho, T.A.; Gryzenhout, M.; Wingfield, M.J. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Stud. Mycol. 2004, 50, 313–322. [Google Scholar]
- Chen, S.F.; Pavlic, D.; Roux, J.; Slippers, B.; Xie, Y.J.; Wingfield, M.J.; Zhou, X.D. Characterization of Botryosphaeriaceae from plantation-grown Eucalyptus species in South China. Plant Pathol. 2011, 60, 739–751. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- AbuQamar, S.F.; Moustafa, K.; Tran, L.S. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Saeed, E.E.; Sham, A.; Salmin, Z.; Abdelmowla, Y.; Iratni, R.; El-Tarabily, K.A.; AbuQamar, S.F. Streptomyces globosus UAE1, a potential effective biocontrol agent for black scorch disease in date palm plantations. Front. Microbiol. 2017, 8, 1455. [Google Scholar] [CrossRef] [PubMed]
- Razdan, V.; Sabitha, M. Integrated disease management: Concepts and practices. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Golam Mortuza, M.; Ilag, L.L. Potential for biocontrol of Lasiodiplodia theobromae (Pat.) Griff. & Maubl. in banana fruits by Trichoderma species. Biol. Control 1999, 15, 235–240. [Google Scholar]
- Adeniyi, D.O.; Adedeji, A.R.; Oduwaye, O.F.; Kolawole, O.O. Evaluation of Biocontrol agents against Lasiodiplodia theobromae causing inflorescence blight of cashew in Nigeria. IOSR J. Agric. Vet. Sci. 2013, 5, 46–48. [Google Scholar]
- Sultana, N.; Ghaffar, A. Effect of fungicides and microbial antagonists in the control of Lasiodiplodia theobromae, the cause of seed rot, seedling and root infection of bottle gourd. Pak. J. Agric. Res. 2010, 23, 46–52. [Google Scholar]
- Syed, R.N.; Mansha, N.; Khaskheli, M.A.; Khanzada, M.A.; Lodhi, A.M. Chemical control of stem end rot of mango caused by Lasiodiplodia theobromae. Pak. J. Phytopathol. 2014, 26, 201–206. [Google Scholar]
- Rehman, A.U.; Naqvi, S.; Latif, M.; Khan, S.; Malik, M.; Freed, S. Emerging resistance against different fungicides in Lasiodiplodia theobromae, the cause of mango dieback in Pakistan. Arch. Biol. Sci. 2015, 67, 241–249. [Google Scholar] [CrossRef]
- Iqbal, Z.; Pervez, M.A.; Ahmad, S.; Iftikhar, Y.; Yasin, M.; Nawaz, A.; Ghazanfar, M.U.; Dasti, A.A.; Saleem, A. Determination of minimum inhibitory concentrations of fungicides against fungus Fusarium mangiferae. Pak. J. Bot. 2010, 42, 3525–3532. [Google Scholar]
- Khan, S.H.; Idrees, M.; Muhammad, F.; Mahmood, A.; Zaidi, S.H. Incidence of shisham (Dalbergia sissoo Roxb.) decline and in vitro response of isolated fungus spp. to various fungicides. Int. J. Agric. Biol. 2004, 6, 611–614. [Google Scholar]
- Saeed, E.E.; Sham, A.; El-Tarabily, K.A.; Abu Elsamen, F.; Iratni, R.; AbuQamar, S.F. Chemical control of dieback disease on date palm caused by the fungal pathogen, Thielaviopsis punctulata, in United Arab Emirates. Plant Dis. 2016, 100, 2370–2376. [Google Scholar] [CrossRef]
- Yanase, Y.; Katsuta, H.; Tomiya, K.; Enomoto, M.; Sakamoto, O. Development of a novel fungicide, penthiopyrad. J. Pestic. Sci. 2013, 38, 167–168. [Google Scholar] [CrossRef]
- Sewell, T.R.; Moloney, S.; Ashworth, M.; Ritchie, F.; Mashanova, A.; Huang, Y.J.; Stotz, H.U.; Fitt, B.D.L. Effects of a penthiopyrad and picoxystrobin fungicide mixture on phoma stem canker (Leptosphaeria spp.) on UK winter oilseed rape. Eur. J. Plant Pathol. 2016, 145, 675–685. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; Moustafa, K.; Tran, L.S. ‘Omics’ and plant responses to Botrytis cinerea. Front. Plant Sci. 2016, 7, 1658. [Google Scholar] [CrossRef] [PubMed]
- Sham, A.; Moustafa, K.; Al-Shamisi, S.; Alyan, S.; Iratni, R.; AbuQamar, S. Microarray analysis of Arabidopsis WRKY33 mutants in response to the necrotrophic fungus Botrytis cinerea. PLoS ONE 2017, 12, e0172343. [Google Scholar] [CrossRef] [PubMed]
- Sham, A.; Al-Azzawi, A.; Al-Ameri, S.; Al-Mahmoud, B.; Awwad, F.; Al-Rawashdeh, A.; Iratni, R.; AbuQamar, S.F. Transcriptome analysis reveals genes commonly induced by Botrytis cinerea infection, cold, drought and oxidative stresses in Arabidopsis. PLoS ONE 2014, 9, e113718. [Google Scholar] [CrossRef] [PubMed]
- Kirsop, B.E.; Doyle, A. Maintenance of Microorganisms and Cultured Cells, a Manual of Laboratory Methods, 2nd ed.; Academic Press: London, UK, 1991. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–555. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Rungjindamai, N. Isolation and evaluation of biocontrol agents in controlling anthracnose disease of mango in Thailand. J. Plant Prot. Res. 2016, 56, 306–311. [Google Scholar] [CrossRef]
- Amponsah, N.T.; Jones, E.; Ridgway, H.J.; Jaspers, M.V. Evaluation of fungicides for the management of Botryosphaeria dieback diseases of grapevines. Pest. Manag. Sci. 2012, 68, 676–683. [Google Scholar] [CrossRef] [PubMed]







| Treatment | DSI 1 | |
|---|---|---|
| 4 Wpt | 12 Wpt | |
| Lt | 3.42 (b) | 4.42 (b) |
| CT | 1.58 (a) | 0.42 (a) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, E.E.; Sham, A.; AbuZarqa, A.; A. Al Shurafa, K.; S. Al Naqbi, T.; Iratni, R.; El-Tarabily, K.; F. AbuQamar, S. Detection and Management of Mango Dieback Disease in the United Arab Emirates. Int. J. Mol. Sci. 2017, 18, 2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102086
Saeed EE, Sham A, AbuZarqa A, A. Al Shurafa K, S. Al Naqbi T, Iratni R, El-Tarabily K, F. AbuQamar S. Detection and Management of Mango Dieback Disease in the United Arab Emirates. International Journal of Molecular Sciences. 2017; 18(10):2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102086
Chicago/Turabian StyleSaeed, Esam Eldin, Arjun Sham, Ayah AbuZarqa, Khawla A. Al Shurafa, Tahra S. Al Naqbi, Rabah Iratni, Khaled El-Tarabily, and Synan F. AbuQamar. 2017. "Detection and Management of Mango Dieback Disease in the United Arab Emirates" International Journal of Molecular Sciences 18, no. 10: 2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102086