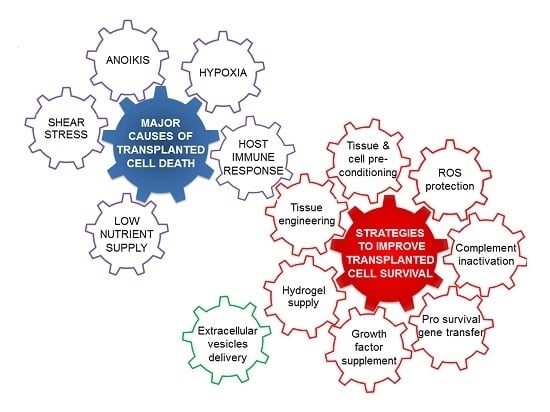
Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies
Abstract
:1. Introduction
2. Challenges to Successful Mesenchymal Stromal Cell Transplantation
2.1. Cell–Extracellular Matrix Interactions
2.2. Mechanical Stress
2.3. Hypoxia and Nutritional Stress
2.4. Immune Response
3. Strategies for Successful Stem Cell Transplantation
3.1. Tissue Engineering: Co-Delivery of Extracellular Matrix Molecules
Cell-Assisted Lipotransfer
3.2. Hydrogel Microcarriers to Reduce Mechanical Stress During Cell Administration
3.3. Preconditioning to Improve Cell Resistance to Stressful Stimuli
3.3.1. Thermal Preconditioning
3.3.2. Hypoxic Preconditioning
3.3.3. Acidic Preconditioning
3.3.4. Nutrient Deprivation Preconditioning
3.3.5. Pharmacologic Cell Preconditioning
3.4. Tissue Preconditioning to Make Recipient Site More Receptive to Donor Cells
3.5. Cell Co-Transplantation with Active Biomaterials for Oxidative Stress Protection
3.6. Genetic Engineering to Improve Cell Survival
3.7. Providing Nutrient Support to Transplanted Cells
3.8. Ex Vivo Expansion in Xeno-Free Media to Reduce the Risk of Immunological Reaction
3.9. Counteracting Complementary Activation
3.10. The Alternative Approach of Cell-Free Therapy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| MSCs | Mesenchymal stromal cells |
| ROS | Reactive oxygen species |
| EVs | Extracellular vesicles |
References
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q. The promise and perils of stem cell therapeutics. Cell Stem Cell 2012, 10, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Copland, I.B.; Galipeau, J. Death and inflammation following somatic cell transplantation. Semin. Immunopathol. 2011, 33, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Rohban, R.; Pieber, T.R. Mesenchymal stem and progenitor cells in regeneration: Tissue specificity and regenerative potential. Stem Cells Int. 2017, 2017, 5173732. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sage, E.K.; Thakrar, R.M.; Janes, S.M. Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy 2016, 18, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Hagenhoff, A.; Bruns, C.J.; Zhao, Y.; von Lüttichau, I.; Niess, H.; Spitzweg, C.; Nelson, P.J. Harnessing mesenchymal stem cell homing as an anticancer therapy. Expert Opin. Biol. Ther. 2016, 16, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Lam, P.Y. Recent discoveries concerning the tumor-mesenchymal stem cell interactions. Biochim. Biophys. Acta 2016, 1866, 290–299. [Google Scholar]
- Dimmeler, S.; Leri, A. Aging and disease as modifiers of efficacy of cell therapy. Circ. Res. 2008, 102, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rando, T.A. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 2011, 193, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid. Med. Cell Longev. 2015, 2015, 309169. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016, 2016, 21522435. [Google Scholar] [CrossRef] [PubMed]
- Efimenko, A.Y.; Kochegura, T.N.; Akopyan, Z.A.; Parfyonova, Y.V. Autologous stem cell therapy: How aging and chronic diseases affect stem and progenitor cells. Biores. Open Access 2015, 4, 26–38. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Xu, Y.; Du, W.; Qi, X.; Liang, L.; Wang, Y.; Feng, G.; Fan, Y.; Han, Z.; Kong, D.; et al. Extracellular matrix can recover the downregulation of adhesion molecules after cell detachment and enhance endothelial cell engraftment. Sci. Rep. 2015, 5, 10902. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Horn, D.; Schup-Magoffin, P.J.; Christman, K.L. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012, 8, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Zouani, O.F.; Kalisky, J.; Ibarboure, E.; Durrieu, M.C. Effect of Bmp-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials 2013, 34, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Yim, H.G.; Choi, Y.J.; Kang, B.J.; Kim, J.; Kwon, S.M.; Kim, B.S.; Hwang, N.S.; Cho, J.Y. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release 2014, 187, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Briquez, P.S.; Güç, E.; Tortelli, F.; Kilarski, W.W.; Metzger, S.; Rice, J.J.; Kuhn, G.A.; Müller, R.; Swartz, M.A.; et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 2014, 343, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 2011, 3, 100ra89. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.A.; Jimenez, F.; Gerber, M.H.; Aroom, K.R.; Shah, S.K.; Harting, M.T.; Gill, B.S.; Savitz, S.I.; Cox, C.S. Effect of needle diameter and flow rate on rat and human mesenchymal stromal cell characterization and viability. Tissue Eng. Part C Methods 2010, 16, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H.; Rose, F.R.; White, L.J.; Shakesheff, K.M. A detailed assessment of varying ejection rate on delivery efficiency of mesenchymal stem cells using narrow-bore needles. Stem Cells Transl. Med. 2016, 5, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H.; White, L.J.; Shakesheff, K.M. The effect of injection using narrow-bore needles on mammalian cells: Administration and formulation considerations for cell therapies. J. Pharm. Pharmacol. 2015, 67, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. Rev. 2010, 26, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, I.; Gadjanski, I.; Vlaski-Lafarge, M.; Buzanska, L.; Loncaric, D.; Sarnowska, A.; Rodriguez, L.; Sandvig, A.; Ivanovic, Z. Strategies to enhance implantation and survival of stem cells after their injection in ischemic neural tissue. Stem Cells Dev. 2017, 26, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, M.; Oudina, K.; David, B.; Myrtil, V.; Collet, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J. Cell. Mol. Med. 2011, 15, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Jahr, H.; van Osch, G.J.; Farrell, E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: Considerations for regenerative medicine approaches. Tissue Eng. Part B Rev. 2010, 16, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yasuda, T.; Weisel, R.D.; Li, R.K. Enhanced cell transplantation: Preventing apoptosis increases cell survival and ventricular function. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H939–H947. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.E.; Ezquer, M.E.; Vicencio, J.M.; Calligaris, S.D. Two complementary strategies to improve cell engraftment in mesenchymal stem cell-based therapy: Increasing transplanted cell resistance and increasing tissue receptivity. Cell Adh. Migr. 2017, 11, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Kasim, N.H.; Rahman, M.T. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int. J. Biol. Sci. 2015, 11, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Rasmusson-Duprez, I.; von Bahr, L.; Connolly-Andersen, A.M.; Elgue, G.; Funke, L.; Hamad, O.A.; Lönnies, H.; Magnusson, P.U.; Sanchez, J.; et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012, 30, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fung, J.; Lin, F. Local inhibition of complement improves mesenchymal stem cell viability and function after administration. Mol. Ther. 2016, 24, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.A.; Udalova, D.V.; Pliss, M.G.; Galagudza, M.M. Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Jung, Y.; Kim, S.H. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention, and engraftment for tissue repair. Biomaterials 2016, 90, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.J.; Vandenburgh, H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008, 2, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hiew, V.V.; Simat, S.F.B.; Teoh, P.L. The advancement of biomaterials in regulating stem cell fate. Stem Cell Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Chen, C.; Hasani-Sadrabadi, M.M.; Yu, B.; Zadeh, H.H.; Wu, B.M.; Moshaverinia, A. Hydrogel elasticity and microarchitecture regulate dental-derived mesenchymal stem cells-host immune system cross-talk. Acta Biomater. 2017, 60, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, A.M.; Noce, V.; Battistelli, C.; Marchetti, A.; Grassi, G.; Cicchini, C.; Tripodi, M.; Amicone, L. Modulating the substrate stiffness to manipulate differentiation of resident liver stem cells and to improve the differentiation state of hepatocytes. Stem Cells Int. 2016, 2016, 5481493. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Gao, P.L.; Wang, K.; Wang, J.Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Mebarki, M.; Coquelin, L.; Layrolle, P.; Battaglia, S.; Tossou, M.; Hernigou, P.; Rouard, H.; Chevallier, N. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater. 2017, 59, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Trivisonno, A.; Di Rocco, G.; Cannistra, C.; Finocchi, V.; Farr, S.; Monti, M.; Toietta, G. Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet. Surg. J. 2014, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Kølle, S.F.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.L.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L.; Gerecht, S. To serve and protect: Hydrogels to improve stem cell-based therapies. Cell Stem Cell 2016, 18, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.A.; Marquardt, L.M.; Heilshorn, S.C. The diverse roles of hydrogel mechanics in injectable stem cell transplantation. Curr. Opin. Chem. Eng. 2017, 15, 15–23. [Google Scholar] [CrossRef]
- Wagner, M.A.; Marks, W.H.; Bhatia, S.K. Hydrogel encapsulation to improve cell viability during syringe needle flow. J. Long Term Eff. Med. Implants 2014, 24, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Ma, T.; Li, Y. Preconditioning stem cells for in vivo delivery. Biores. Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Jahanian-Najafabadi, A.; Roudkenar, M.H. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments: In vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones 2015, 20, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.K.H.; Ashraf, M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J. Mol. Cell. Cardiol. 2008, 45, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Wei, Z.; Wei, L. Preconditioning strategy in stem cell transplantation therapy. Transl. Stroke Res. 2013, 4, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.K.H.; Ashraf, M. Preconditioning and stem cell survival. J. Cardiovasc. Transl. Res. 2010, 3, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.F.; Yao, L.; Zhang, X.C.; Li, G.D.; Wu, D.Q. Heat shock pretreatment improves stem cell repair following ischemia-reperfusion injury via autophagy. World J. Gastroenterol. 2015, 21, 12822–12834. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Meng, W.; Jegga, A.G.; Wang, Y.; Cai, W.; Kim, H.W.; Pasha, Z.; Wen, Z.; Rao, F.; et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: A critical role for HSF1/mirR-34a/HSP70 pathway. Stem Cells 2014, 32, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.A.; He, A.; Hu, X.; Jiang, Y.; Sun, Y.; Jiang, J.; Gui, C.; Wang, Y.; Chen, H. Anoxic preconditioning: A way to enhance the cardioprotection of mesenchymal stem cells. Int. J. Cardiol. 2009, 133, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, C.; Melchionna, R.; Straino, S.; Romani, M.; Cappuzzello, C.; Annese, V.; Wu, J.C.; Pompilio, G.; Santoni, A.; Gaetano, C.; et al. Ex vivo acidic preconditioning enhances bone marrow ckit+ cell therapeutic potential via increased CXCR4 expression. Eur. Heart J. 2013, 34, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence preconditioned human multipotent stromal cells adopt a metabolic profile favorable for enhanced survival under ischemia. Stem Cells 2017, 35, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Smolenski, R.T.; Jayakumar, J.; Murtuza, B.; Brand, N.J.; Yacoub, M.H. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation 2000, 102, III216–III221. [Google Scholar] [CrossRef] [PubMed]
- Mantel, C.R.; O’Leary, H.A.; Chitteti, B.R.; Huang, X.; Cooper, S.; Hangoc, G.; Brustovetsky, N.; Srour, E.F.; Lee, M.R.; Messina-Graham, S.; et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 2015, 161, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Muscari, C.; Giordano, E.; Bonafè, F.; Govoni, M.; Pasini, A.; Guarnieri, C. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J. Biomed. Sci. 2013, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.T.; Dilley, R.J.; Dusting, G.J.; Lim, S.Y. Ischemic preconditioning for cell-based therapy and tissue engineering. Pharmacol. Ther. 2014, 142, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Beegle, J.; Lakatos, K.; Kalomoiris, S.; Stewart, H.; Isseroff, R.R.; Nolta, J.A.; Fierro, F.A. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015, 33, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, C.; Yu, Z.; Shi, Z.; Dang, X.; Wang, K. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and osteogenesis in rabbit femoral head osteonecrosis. Bone 2015, 81, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Gao, C.Y.; Zhai, Y.P. Adaptive protection against damage of preconditioning human umbilical cord-derived mesenchymal stem cells with hydrogen peroxide. Genet. Mol. Res. 2014, 13, 7304–7317. [Google Scholar] [CrossRef] [PubMed]
- Carrière, A.; Ebrahimian, T.G.; Dehez, S.; Augé, N.; Joffre, C.; André, M.; Arnal, S.; Duriez, M.; Barreau, C.; Arnaud, E.; et al. Preconditioning by mitochondrial reactive oxygen species improves the proangiogenic potential of adipose-derived cells-based therapy. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Haider, H.K.H.; Idris, N.M.; Jiang, S.; Ahmed, R.P.; Ashraf, M. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid. Redox Signal. 2010, 12, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.; Iso, Y.; Sato, C.; Sasai, M.; Spees, J.L.; Toyoda, M.; Maeda, A.; Maezawa, H.; Togo, T.; Nemoto, H.; et al. Priming with erythropoietin enhances cell survival and angiogenic effect of mesenchymal stem cell implantation in rat limb ischemia. Regen. Ther. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Wisel, S.; Khan, M.; Kuppusamy, M.L.; Mohan, I.K.; Chacko, S.M.; Rivera, B.K.; Sun, B.C.; Hideg, K.; Kuppusamy, P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J. Pharmacol. Exp. Ther. 2009, 329, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, J.; Wang, Y.; Zhang, Y.; Ii, M.; Shen, Z.; Hui, J. Mesenchymal stem cells preconditioned with trimetazidine promote neovascularization of hearts under hypoxia/reoxygenation injury. Int. J. Clin. Exp. Med. 2015, 8, 16991–17005. [Google Scholar] [PubMed]
- Sun, Y.; Li, Q.F.; Yan, J.; Hu, R.; Jiang, H. Isoflurane preconditioning promotes the survival and migration of bone marrow stromal cells. Cell. Physiol. Biochem. 2015, 36, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Léniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef] [PubMed]
- Nouri, F.; Salehinejad, P.; Nematollahi-Mahani, S.N.; Kamarul, T.; Zarrindast, M.R.; Sharifi, A.M. Deferoxamine preconditioning of neural-like cells derived from human Wharton's jelly mesenchymal stem cells as a strategy to promote their tolerance and therapeutic potential: An in vitro study. Cell. Mol. Neurobiol. 2016, 36, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Wang, J.A.; Ji, X.Y.; Yu, S.P.; Wei, L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res. Ther. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, N.; Borie, M.; Desnoyers, A.; Menaouar, A.; Stevens, L.M.; Mansour, S.; Danalache, B.A.; Roy, D.C.; Jankowski, M.; Gutkowska, J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology 2012, 153, 5361–5372. [Google Scholar] [CrossRef] [PubMed]
- Der Sarkissian, S.; Lévesque, T.; Noiseux, N. Optimizing stem cells for cardiac repair: Current status and new frontiers in regenerative cardiology. World J. Stem Cells 2017, 9, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Khanlarkhani, N.; Sabbaghziarani, F.; Nekoonam, S.; Majidpoor, J.; Hosseini, A.; Pasbakhsh, P.; Kashani, I.R.; Zendedel, A. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mias, C.; Trouche, E.; Seguelas, M.H.; Calcagno, F.; Dignat-George, F.; Sabatier, F.; Piercecchi-Marti, M.D.; Daniel, L.; Bianchi, P.; Calise, D.; et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells 2008, 26, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, J.; Lv, B.; Yu, B. Nicorandil protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Int. J. Mol. Med. 2015, 36, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, F.; Wang, L.; Zhang, G.; Wang, Z.; Chen, J.; Gao, X. Lipopolysaccharide preconditioning enhances the efficacy of mesenchymal stem cells transplantation in a rat model of acute myocardial infarction. J. Biomed. Sci. 2009, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, D.; Togashi, I.; Miyoshi, S.; Nishiyama, N.; Hida, N.; Tsuji, H.; Tsuruta, H.; Segawa, K.; Tsukada, Y.; Ogawa, S.; et al. Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells 2011, 29, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Li, T.; Yuan, L.; Liu, H.; Wang, X.; Wang, F.; Wang, S.; Hao, A.; Liu, D.; et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 2016, 7, 58089–58104. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Rinaldi, L.; Rossi, C.; Geng, Y.J.; De Caterina, R. Prostacyclin improves transcoronary myocardial delivery of adipose tissue-derived stromal cells. Eur. Heart J. 2006, 27, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Walter, D.H.; Seeger, F.H.; Leistner, D.M.; Steiner, J.; Ziegler, I.; Lutz, A.; Khaled, W.; Klotsche, J.; Tonn, T.; et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: The CELLWAVE randomized clinical trial. JAMA 2013, 309, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Retuerto, M.A.; Schalch, P.; Patejunas, G.; Carbray, J.; Liu, N.; Esser, K.; Crystal, R.G.; Rosengart, T.K. Angiogenic pretreatment improves the efficacy of cellular cardiomyoplasty performed with fetal cardiomyocyte implantation. J. Thorac. Cardiovasc. Surg. 2004, 127, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Muraca, M.; Ferraresso, C.; Vilei, M.T.; Granato, A.; Quarta, M.; Cozzi, E.; Rugge, M.; Pauwelyn, K.A.; Caruso, M.; Avital, I.; et al. Liver repopulation with bone marrow derived cells improves the metabolic disorder in the Gunn rat. Gut 2007, 56, 1725–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.; Yu, T.; Huang, K.; Lu, J.; Zhang, H.; Hu, S. Remote ischemic postconditioning ameliorates the mesenchymal stem cells engraftment in reperfused myocardium. PLoS ONE 2016, 11, e0146074. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, B.R.; Gupta, M.K.; Martin, J.R.; Duvall, C.L. Reactive oxygen species shielding hydrogel for the delivery of adherent and nonadherent therapeutic cell types. Tissue Eng. Part A 2017. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, N.; Leijten, J.; Camci-Unal, G.; Hjortnaes, J.; Ribas, J.; Paul, A.; Mostafalu, P.; Gaharwar, A.K.; Qiu, Y.; Sonkusale, S.; et al. Oxygen-generating photo-cross-linkable hydrogels support cardiac progenitor cell survival by reducing hypoxia-induced necrosis. ACS Biomat. Sci. Eng. 2016, 151. [Google Scholar] [CrossRef]
- Park, J.S.; Suryaprakash, S.; Lao, Y.H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Gentile, A.; Antonini, A.; Truffa, S.; Piaggio, G.; Capogrossi, M.; Toietta, G. Analysis of biodistribution and engraftment into the liver of genetically modified mesenchymal stromal cells derived from adipose tissue. Cell Transplant. 2012, 21, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Penn, M.S.; Mangi, A.A. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ. Res. 2008, 102, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Haider, H.K.H.; Idris, N.M.; Salim, A.; Ashraf, M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ. Res. 2006, 99, 776–784. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.M.; McMahon, J.; Stocca, A.; Duffy, A.; Flynn, A.; O’Toole, D.; O’Brien, T. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum. Gene Ther. 2013, 24, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Trivisonno, A.; Samengo, D.; Pani, G.; Toietta, G. Promotion of survival and engraftment of transplanted adipose tissue-derived stromal and vascular cells by overexpression of manganese superoxide dismutase. Int. J. Mol. Sci. 2016, 17, 1082. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chang, W.; Lim, S.; Seo, H.S.; Shim, C.Y.; Park, S.; Yoo, K.J.; Kim, B.S.; Min, B.H.; Lee, H.; et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells 2007, 25, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Youn, S.W.; Cheon, S.I.; Kim, T.Y.; Hur, J.; Zhang, S.Y.; Lee, S.P.; Park, K.W.; Lee, M.M.; Choi, Y.S.; et al. Regulation of endothelial cell and endothelial progenitor cell survival and vasculogenesis by integrin-linked kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, E.; Cha, M.J.; Hwang, K.C. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: A prerequisite for cell therapy. Oxid. Med. Cell. Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Gentile, A.; Antonini, A.; Ceradini, F.; Wu, J.; Capogrossi, M.; Toietta, G. Enhanced healing of diabetic wounds by topical administration of adipose tissue-derived stromal cells overexpressing stromal-derived factor-1: Biodistribution and engraftment analysis by bioluminescent imaging. Stem Cells Int. 2011, 2011, 304562. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Walczak, P.; Lukomska, B.; Janowski, M. Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cells Int. 2016, 2016, 4956063. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Z.; Guo, J.; Ni, A.; Deb, A.; Zhang, L.; Mirotsou, M.; Pratt, R.E.; Dzau, V.J. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ. Res. 2010, 106, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ou, L.; Zhou, X.; Li, F.; Jia, X.; Zhang, Y.; Liu, X.; Li, Y.; Ward, C.A.; Melo, L.G.; et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008, 16, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Dakhlallah, D.; Zhang, J.; Yu, L.; Marsh, C.B.; Angelos, M.G.; Khan, M. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J. Cardiovasc. Pharmacol. 2015, 65, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, M.; Nguyen, P.K.; Gong, Y.; Li, Z.; Jia, F.; Lan, F.; Liu, J.; Nag, D.; Robbins, R.C.; et al. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation 2011, 124, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Z.; Lin, L.; Fu, M.; Gao, Y.; Shen, Y.; Zou, Y.; Sun, A.; Qian, J.; Ge, J. MiR-210 over-expression enhances mesenchymal stem cell survival in an oxidative stress environment through antioxidation and c-Met pathway activation. Sci. China Life Sci. 2014, 57, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Wu, X.; Wei, S.; Han, W.; Lin, J.; Kang, M.; Chen, L. Hepatocyte growth factor-modified mesenchymal stem cells improve ischemia/reperfusion-induced acute lung injury in rats. Gene Ther. 2017, 24, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kwon, K.; Lim, S.; Kang, S.M.; Ko, Y.G.; Xu, Z.; Chung, J.H.; Kim, B.S.; Lee, H.; Joung, B.; et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol. Cells 2005, 19, 402–407. [Google Scholar] [PubMed]
- Liu, N.; Zhang, Y.; Fan, L.; Yuan, M.; Du, H.; Cheng, R.; Liu, D.; Lin, F. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by survivin on experimental stroke in rats. J. Transl. Med. 2011, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Tang, Y.; Zhang, Y.C.; Qian, K.; Shen, L.; Phillips, M.I. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005, 46, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Taylor, D.A.; Geng, Y.J.; De Caterina, R.; Shelat, H.; Perin, E.C.; Willerson, J.T. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ. Res. 2013, 113, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Kutschka, I.; Kofidis, T.; Chen, I.Y.; von Degenfeld, G.; Zwierzchoniewska, M.; Hoyt, G.; Arai, T.; Lebl, D.R.; Hendry, S.L.; Sheikh, A.Y.; et al. Adenoviral human Bcl-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation 2006, 114, I174–I180. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Chen, J.; Antus, B.; Su, F.; Wang, M.; Exton, M.S. Adenovirus-mediated Bcl-2 gene transfer inhibits apoptosis and promotes survival of allogeneic transplanted hepatocytes. Surgery 2001, 130, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.B.; Fedak, P.W.; Weisel, R.D.; Yasuda, T.; Kiani, G.; Mickle, D.A.; Jia, Z.Q.; Li, R.K. Enhanced IGF-1 expression improves smooth muscle cell engraftment after cell transplantation. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2840–H2849. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Khan, M.; Toko, H.; Bailey, B.; Cottage, C.T.; Wallach, K.; Nag, D.; Lee, A.; Siddiqi, S.; Lan, F.; et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol. 2012, 60, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Muir, L.A.; Murry, C.E.; Chamberlain, J.S. Prosurvival factors improve functional engraftment of myogenically converted dermal cells into dystrophic skeletal muscle. Stem Cells Dev. 2016, 25, 1159–1569. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Tajima, S.; Mizuno, H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: Stem cell transplantation methods that enhance stemness. Stem Cell. Res. Ther. 2015, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Tharp, K.M.; Ye, J.; Santiago-Ortiz, J.L.; Jackson, W.M.; Stahl, A.; Schaffer, D.V.; Yeghiazarians, Y.; Healy, K.E. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials 2015, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Gregory, C.A.; Singh, H.; Tucker, H.A.; Peister, A.; Lynch, P.J.; Hsu, S.C.; Smith, J.; Prockop, D.J. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol. Ther. 2004, 9, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, W.; Zhang, L.; Fung, J.; Lin, F. Painting factor H onto mesenchymal stem cells protects the cells from complement- and neutrophil-mediated damage. Biomaterials 2016, 102, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood 2012, 120, 3436–3443. [Google Scholar] [CrossRef] [PubMed]
- Soland, M.A.; Bego, M.; Colletti, E.; Zanjani, E.D.; St., Jeor, S.; Porada, C.D.; Almeida-Porada, G. Mesenchymal stem cells engineered to inhibit complement-mediated damage. PLoS ONE 2013, 8, e60461. [Google Scholar] [CrossRef] [PubMed]
- Doorn, J.; Moll, G.; Le Blanc, K.; van Blitterswijk, C.; de Boer, J. Therapeutic applications of mesenchymal stromal cells: Paracrine effects and potential improvements. Tissue Eng. Part B Rev. 2012, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell. Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell. Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Koppers-Lalic, D.; Hogenboom, M.M.; Middeldorp, J.M.; Pegtel, D.M. Virus-modified exosomes for targeted RNA delivery; A new approach in nanomedicine. Adv. Drug Deliv. Rev. 2013, 65, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.; Schiffelers, R.M.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016, 111, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Sahler, J.; Woeller, C.F.; Phipps, R.P. Microparticles engineered to highly express peroxisome proliferator-activated receptor-γ decreased inflammatory mediator production and increased adhesion of recipient monocytes. PLoS ONE 2014, 9, e113189. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell. Transplant. 2013, 22, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory affects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell. Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Microvesicles from mesenchymal stromal cells protect against acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell. Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Svensson, K.J.; Belting, M. Exosome and microvesicle mediated phene transfer in mammalian cells. Semin. Cancer Biol. 2014, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.A.; van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, L.; Battistelli, C.; Montaldo, C.; Citarella, F.; Strippoli, R.; Cicchini, C. Functional roles and therapeutic applications of exosomes in hepatocellular carcinoma. Biomed. Res. Int. 2017, 2017, 2931813. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genomics Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Deregibus, M.C.; Camussi, G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol. Lett. 2015, 168, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Jorgensen, C.; Noël, D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013, 95, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Luciano, R.; Saracino, R.; Battafarano, G.; Rizzo, C.; Pascucci, L.; Alessandri, G.; Pessina, A.; Perrotta, A.; Fierabracci, A.; et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin. Biol. Ther. 2015, 15, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards therapeutic delivery of extracellular vesicles: Strategies for in vivo tracking and biodistribution analysis. Stem Cells Int. 2016, 2016, 5029619. [Google Scholar] [CrossRef]
- Shin, H.; Ryu, H.H.; Kwon, O.; Park, B.S.; Jo, S.J. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. Int. J. Dermatol. 2015, 54, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Muraca, M.; Piccoli, M.; Franzin, C.; Tolomeo, A.M.; Jurga, M.; Pozzobon, M.; Perilongo, G. Diverging concepts and novel perspectives in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1021. [Google Scholar] [CrossRef] [PubMed]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 2015, 11, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
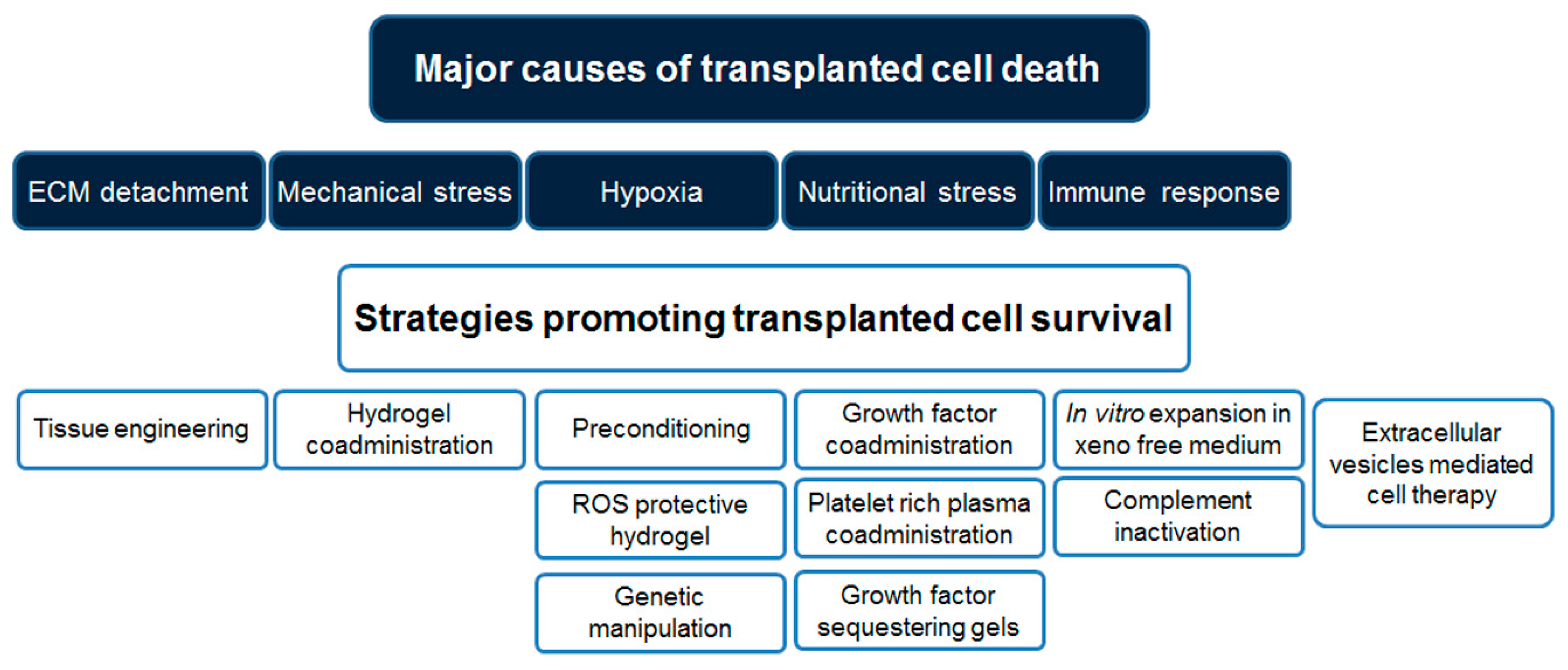
| Conditioning Method | Reference |
|---|---|
| Thermal | [62,63] |
| Hypoxic | [32,64] |
| Anoxic | [65] |
| Acidic | [66] |
| Nutrient deprivation | [67] |
| Drug Name | Drug Function | Reference |
|---|---|---|
| Trimetazidine | Cytoprotective, anti-ischemic | [78,79] |
| Isoflurane | Cytoprotective | [80] |
| Erythropoietin | Anti-apoptotic | [77] |
| Deferoxamine: | HIF-1α stabilizer | [81,82] |
| Dimethyloxalylglycine | HIF-1α stabilizer | [83] |
| Antimycin | Mitochondrial inhibitor | [75] |
| Oxytocin | Anti-oxidant | [84] |
| Celastrol | Anti-oxidant | [85] |
| Melatonin | Anti-oxidant | [86,87] |
| Nicorandil | K+ channel activator | [88] |
| Diazoxide | K+ channel activator | [76] |
| Lipopolysaccharide | TRL4 agonist | [89] |
| Pioglitazone | PPAR-γ agonists | [90] |
| NaHS | H2S donor | [91] |
| Cell Type | Gene Name | Gene Function | Reference |
|---|---|---|---|
| MSC | Akt | Anti-apoptotic | [117] |
| HGF | Anti-apoptotic | [118] | |
| Akt and Ang-1 | Anti-apoptotic/angiogenesis | [103] | |
| FGF-2 | Pro-survival | [119] | |
| Hsp27 | Pro-survival | [104] | |
| Survivin | Pro-survival | [120] | |
| HO-1 | Anti-oxidant | [121] | |
| SOD-2 | Anti-oxidant | [105] | |
| tTG | Promote cell adhesion | [106] | |
| CCR-1 | Promote cell homing | [112] | |
| CXCR4 | Promote cell homing | [113] | |
| SDF-1 | Promote cell homing | [110] | |
| TERT | Telomerase | [122] | |
| miR-21, -24, -221 | Pro-survival | [115] | |
| miR-133a | Pro-survival | [114] | |
| miR-210 | Pro-survival | [116] | |
| CM; SMCs; HEP | Bcl-2 | Anti-apoptotic | [33,123,124] |
| SMC | IGF-1 | Pro-survival | [125] |
| EPC | ILK-1 | Promote cell adhesion | [107] |
| CPC | Pim-1 | Anti-apoptotic | [126] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102087
Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. International Journal of Molecular Sciences. 2017; 18(10):2087. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102087
Chicago/Turabian StyleBaldari, Silvia, Giuliana Di Rocco, Martina Piccoli, Michela Pozzobon, Maurizio Muraca, and Gabriele Toietta. 2017. "Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies" International Journal of Molecular Sciences 18, no. 10: 2087. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102087