Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine
Abstract
:1. Introduction
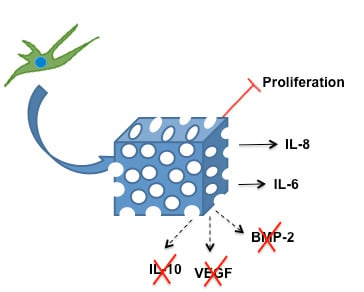
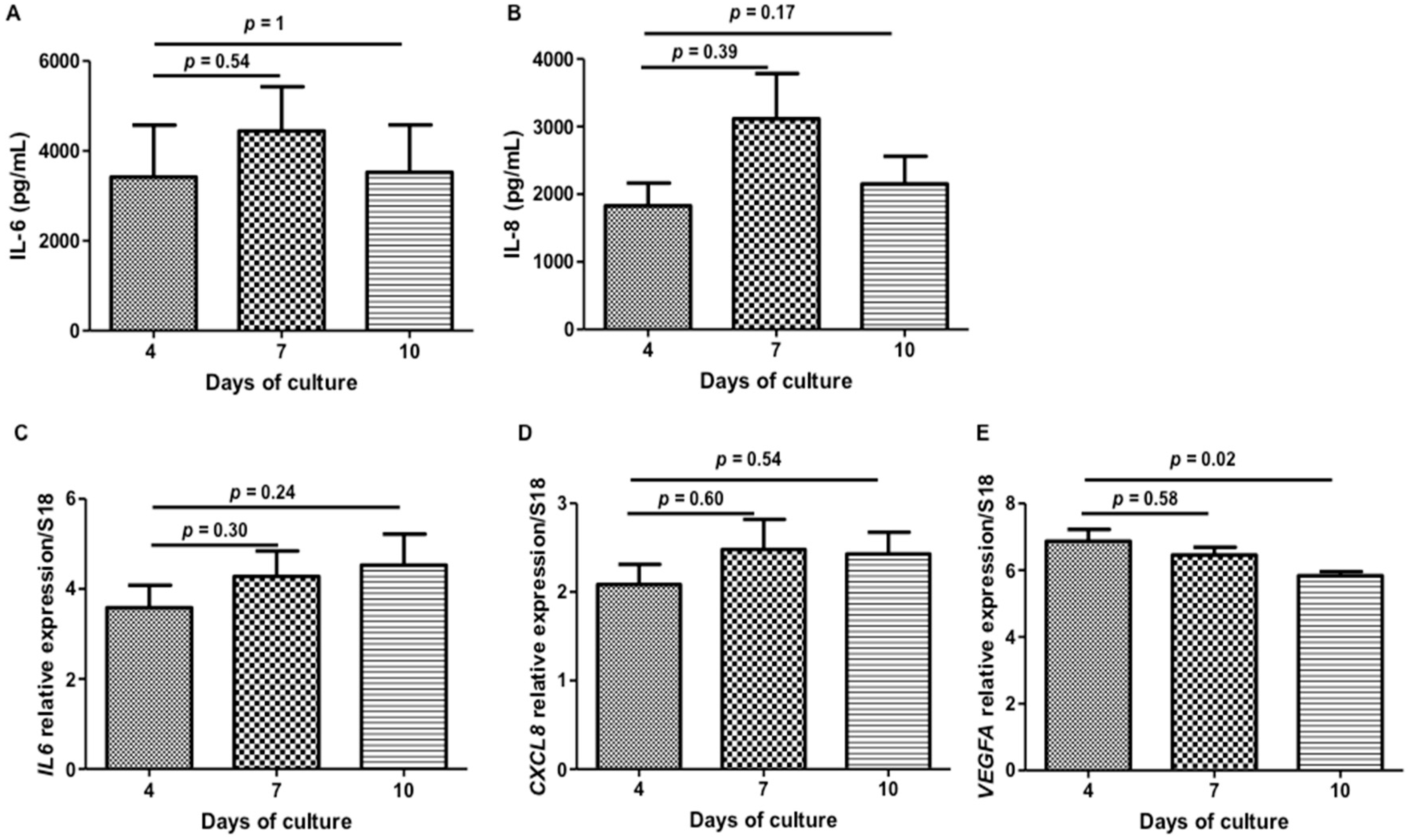
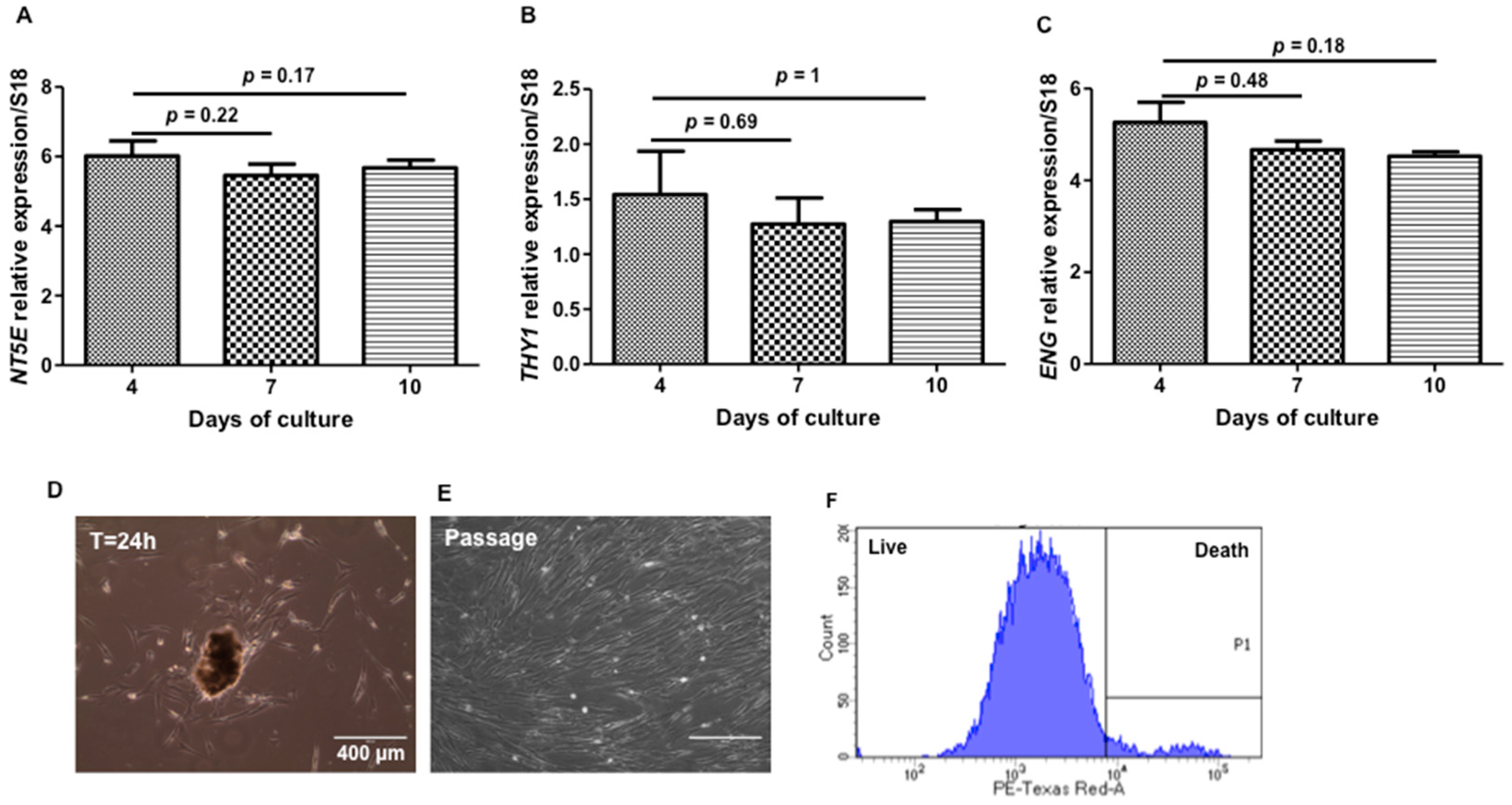
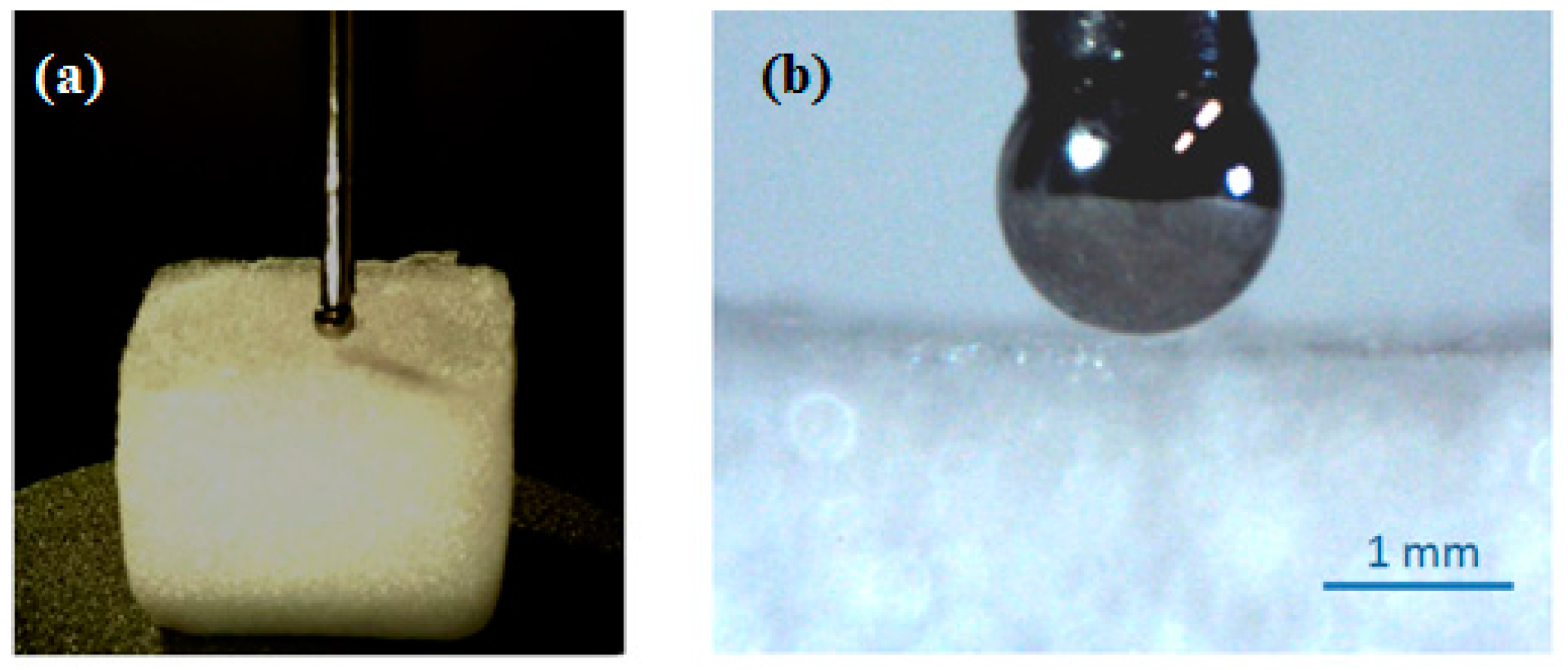
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.1.1. Scanning Electron Microscopy (SEM)
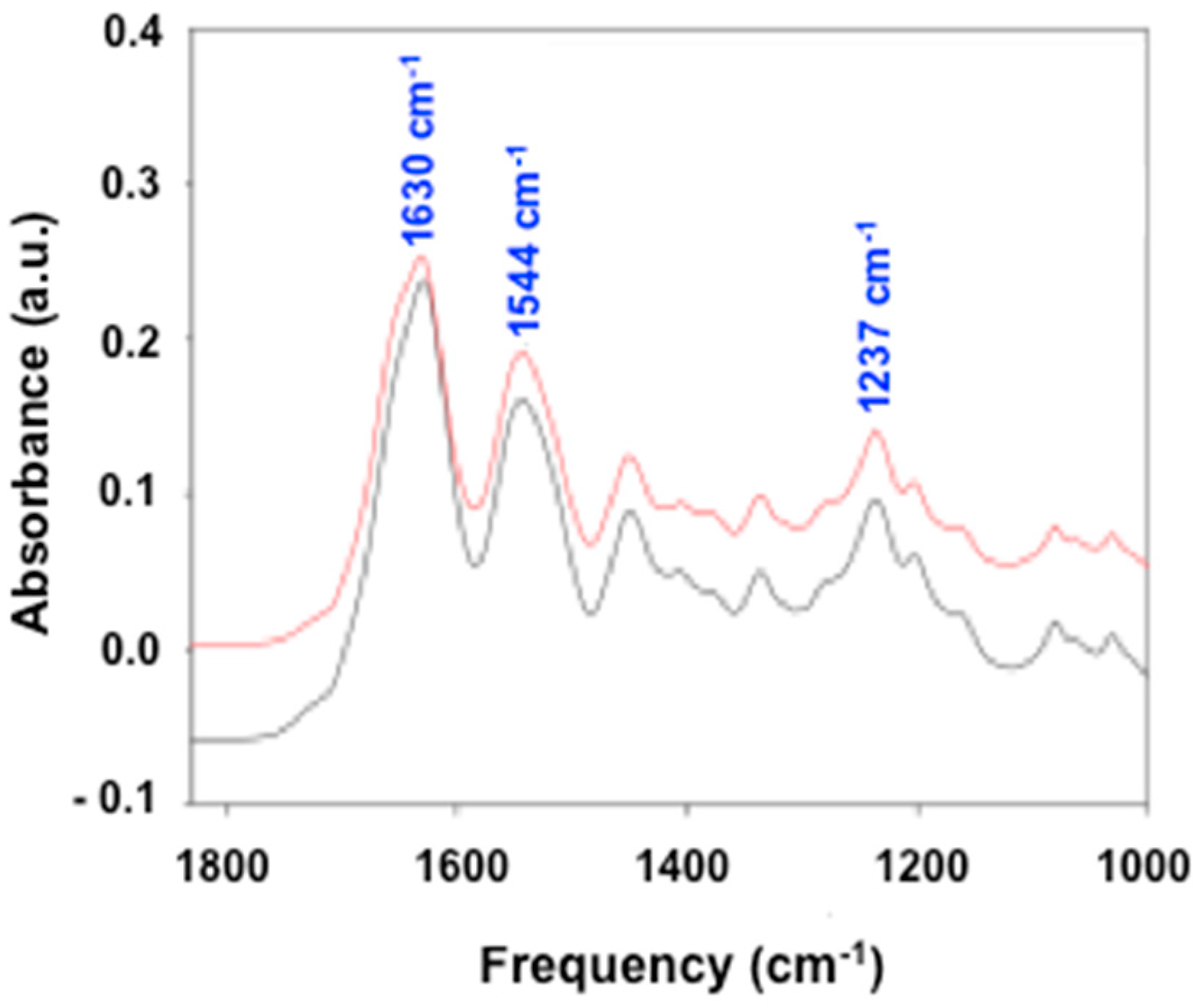
3.1.2. Fourier Transform Infrared (FTIR)
3.1.3. Ion Exchange Chromatography
3.1.4. Mercury Intrusion Porosimetry
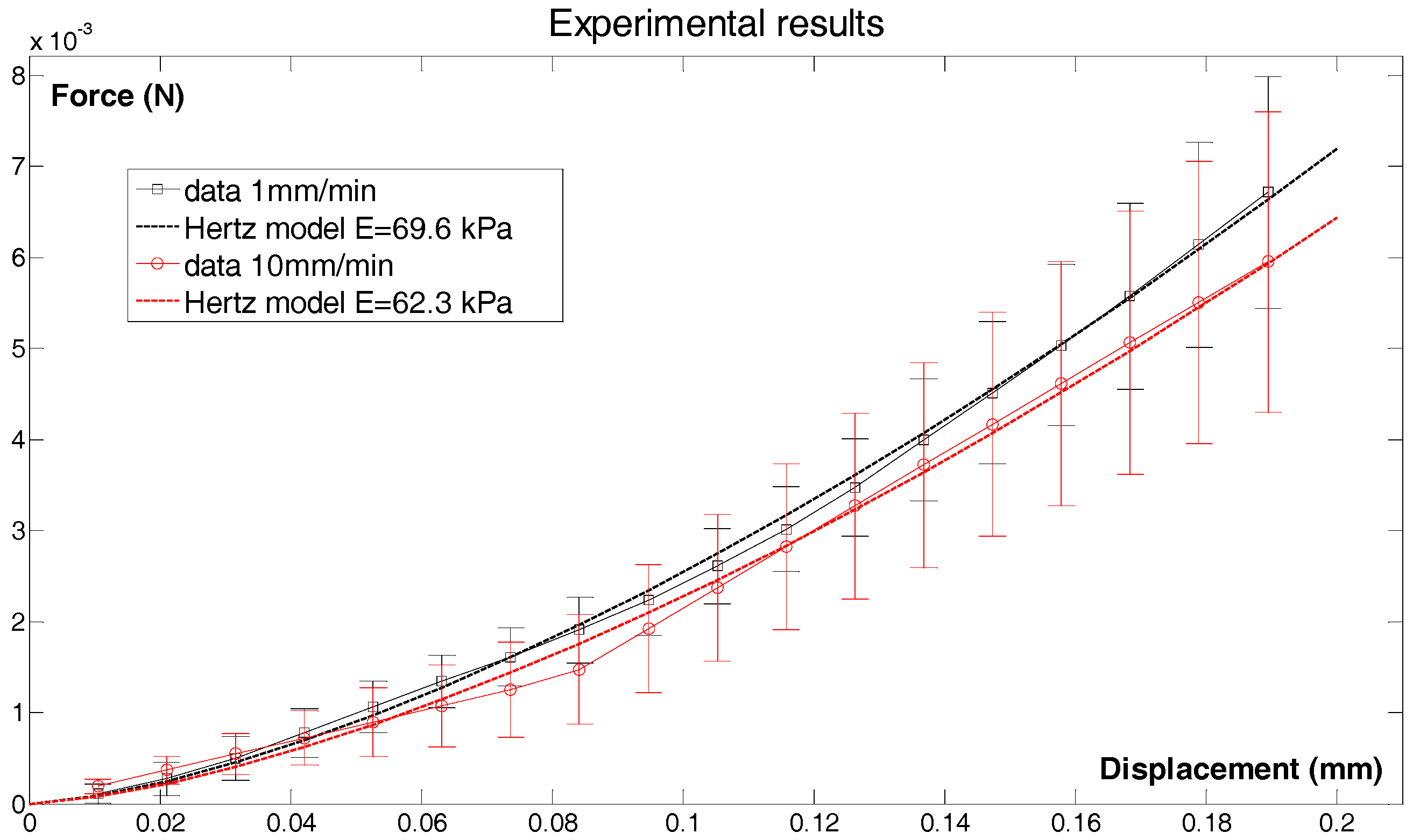
3.1.5. Micro-Indentation
3.2. Biological Experiments
3.2.1. Cell Culture
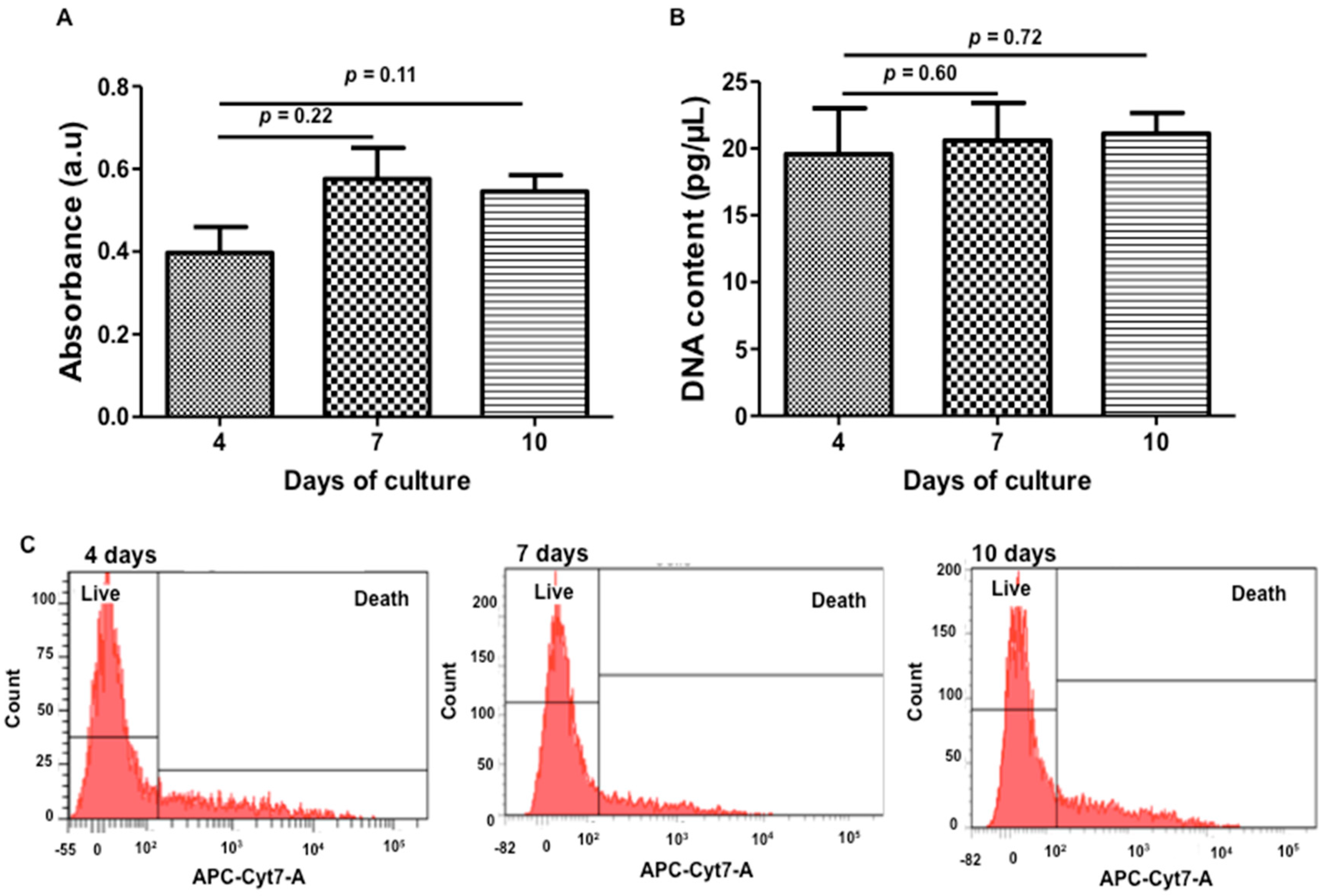
3.2.2. WST-1
3.2.3. DNA Quantification
3.2.4. Zombie® Labeling
3.2.5. SEM
3.2.6. Confocal Laser Scanning Microscopy (CLSM)
3.2.7. Histology and Immunohistochemistry
3.2.8. ELISA Quantification
3.2.9. Chemotaxis Assay
3.2.10. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| MSCs | Mesenchymal Stem Cells |
| WJ-MSCs | Wharton Jelly-Derived Mesenchymal Stem Cells |
| IL | Interleukin |
| SEM | Scanning Electron Microscopy |
| CLSM | Confocal Laser Scanning Microscopy |
| FTIR | Fourier Transform Infrared spectra |
| CD | Cluster of Differentiation |
| qRT-PCR | Quantitative Real Time Polymerase Chain Reaction |
| HES | Hematoxylin-Eosin-Saffron |
| WST-1 | Water-Soluble Tetrazolium Salt-1 |
| DNA | Deoxyribonucleic Acid |
| VEGF | Vascular Endothelial Growth Factor |
| ELISA | Enzyme-Linked Immunosorbent Assay |
References
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell. Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Pfeifer, K.; Petry, F.; Powell, N.; Delzeit, J.; Weiss, M.L. Standardizing umbilical cord mesenchymal stromal cells for translation to clinical use: Selection of GMP-compliant medium and a simplified isolation method. Stem Cells Int. 2016, 2016, e6810980. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.; Harmouch, C.; Lataillade, J.J.; Laurent-Maquin, D.; Labrude, P.; Menu, P.; Kerdjoudj, H. Stem cells: A promising source for vascular regenerative medicine. Stem Cells Dev. 2014, 23, 2931–2949. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. CCS 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xiao, Y.; Liu, C. The horizon of materiobiology: A perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Stachon, S.; Mack, A.; Rohde, M.; Just, L. Evaluation of a thin and mechanically stable collagen cell carrier. Tissue Eng. Part C Methods 2011, 17, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.; Chmielewski, J. Advances in the design and higher-order assembly of collagen mimetic peptides for regenerative medicine. Curr. Opin. Biotechnol. 2017, 46, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Vennat, E.; Bogicevic, C.; Fleureau, J.M.; Degrange, M. Demineralized dentin 3D porosity and pore size distribution using mercury porosimetry. Dent. Mater. 2009, 25, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, W.; Tae, G.; Kim, Y.H.; Kang, S.S.; Kim, S.E.; Jung, Y.; Kim, S.H. Enhanced regeneration of the ligament-bone interface using a poly(l-lactide-co-ε-caprolactone) scaffold with local delivery of cells/BMP-2 using a heparin-based hydrogel. Acta Biomater. 2011, 7, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Mechiche Alami, S.; Velard, F.; Draux, F.; Siu Paredes, F.; Josse, J.; Lemaire, F.; Gangloff, S.C.; Graesslin, O.; Laurent-Maquin, D.; Kerdjoudj, H. Gene screening of Wharton’s jelly derived stem cells. Biomed. Mater. Eng. 2014, 24, 53–61. [Google Scholar] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2016, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhao, Y.; Xiao, Z.; Han, J.; Chen, B.; Chen, L.; Dai, J. The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells. J. Genet. Genom. 2012, 39, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Brenner, M.; Wolujewicz, P.; Zhang, Z.; Mao, Y.; Batish, M.; Kohn, J.; Moghe, P.V. Profiling stem cell states in three-dimensional biomaterial niches using high content image informatics. Acta Biomater. 2016, 45, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Heitz, J.; Plamadeala, C.; Wiesbauer, M.; Freudenthaler, P.; Wollhofen, R.; Jacak, J.; Klar, T.A.; Magnus, B.; Köstner, D.; Weth, A.; et al. Bone-forming cells with pronounced spread into the third dimension in polymer scaffolds fabricated by two-photon polymerization. J. Biomed. Mater. Res. A 2017, 105, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.P.; Liu, Y.S.; Rimando, M.G.; Ho, J.H.; Lin, K.H.; Lee, O.K. Three-dimensional spherical spatial boundary conditions differentially regulate osteogenic differentiation of mesenchymal stromal cells. Sci. Rep. 2016, 6, 21253. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Jang, I.K.; Lee, M.W.; Kim, H.E.; Yang, M.S.; Eom, Y.; Lee, J.E.; Kim, Y.J.; Yang, S.K.; Jung, H.L.; et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009, 259, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, S.H.; Wu, J.; Zang, W.F.; Dhingra, S.; Sun, L.; Weisel, R.D.; Li, R.K. Interleukin-6 downregulation with mesenchymal stem cell differentiation results in loss of immunoprivilege. J. Cell. Mol. Med. 2013, 17, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus tensile measurements of young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Velard, F.; Laurent-Maquin, D.; Braux, J.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.M.; Belaaouaj, A.; Laquerriere, P. The effect of zinc on hydroxyapatite-mediated activation of human polymorphonuclear neutrophils and bone implant-associated acute inflammation. Biomaterials 2010, 31, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.E.; Ezquer, M.E.; Vicencio, J.M.; Calligaris, S.D. Two complementary strategies to improve cell engraftment in mesenchymal stem cell-based therapy: Increasing transplanted cell resistance and increasing tissue receptivity. Cell Adhes. Migr. 2017, 11, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]



| Amino-Acid | HEMOCOLLAGENE® | Type I Collagen (Reference) |
|---|---|---|
| Hydroxyproline | 91.2 | 101.4 |
| Aspartic acid | 49.7 | 49.7 |
| Threonine | 19.4 | 18.9 |
| Serine | 33.7 | 28.8 |
| Glutamic acid | 75.2 | 72.1 |
| Proline | 126.8 | 117.3 |
| Glycine | 322.5 | 327.5 |
| Alanine | 106.5 | 114.8 |
| Valine | 23.8 | 20.9 |
| Methionine | 3.1 | 7.5 |
| Isoleucine | 13.5 | 11.9 |
| Leucine | 26.9 | 25.8 |
| Tyrosine | 2.0 | 2.0 |
| Phenylalanine | 14.7 | 13.9 |
| Hydroxylysine | 6.3 | 6.5 |
| Lysine | 24.6 | 26.3 |
| Histidine | 5.3 | 5 |
| Arginine | 54.6 | 49.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubert, L.; Dubus, M.; Rammal, H.; Bour, C.; Mongaret, C.; Boulagnon-Rombi, C.; Garnotel, R.; Schneider, C.; Rahouadj, R.; Laurent, C.; et al. Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 2210. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102210
Aubert L, Dubus M, Rammal H, Bour C, Mongaret C, Boulagnon-Rombi C, Garnotel R, Schneider C, Rahouadj R, Laurent C, et al. Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine. International Journal of Molecular Sciences. 2017; 18(10):2210. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102210
Chicago/Turabian StyleAubert, Léa, Marie Dubus, Hassan Rammal, Camille Bour, Céline Mongaret, Camille Boulagnon-Rombi, Roselyne Garnotel, Céline Schneider, Rachid Rahouadj, Cedric Laurent, and et al. 2017. "Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine" International Journal of Molecular Sciences 18, no. 10: 2210. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102210