Relation among Aromatase P450 and Tumoral Growth in Human Prolactinomas
Abstract
:1. Introduction
2. Aromatase Expression in Pituitary Gland
3. Estrogens, Prolactin and Aromatase
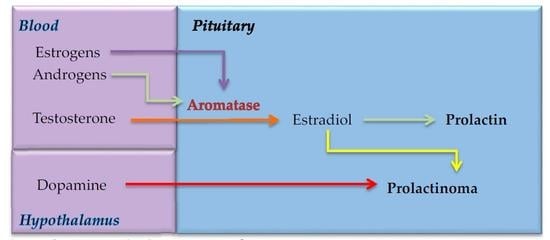
4. Possible Involvement of Aromatase P450 in the Pathogenesis of Prolactinomas
5. Balance among Cell Proliferation and Apoptosis in Prolactinomas
6. Estrogenic Receptors, Prolactinomas, and AIB1
7. P53 and P27 Proteins in Prolactinomas
8. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| LH | Luteinizing hormone |
| PRL | Prolactin |
| VIP | Vasoactive intestinal polypeptide |
| TRH | Thyrotropin-releasing hormone |
| IGF-IR | Insulin-like growth factor 1 receptor |
| EGFR | Epidermal growth factor receptor |
| MAPK | Mitogen-activated protein kinase |
| BAX | Bcl-2-like protein 4 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ER | Estrogenic receptor |
| PTTG | Pituitary tumor transforming gene |
| VEGF | Vascular endothelial growth factor |
| AIB1 | Amplified in breast 1 protein |
| PCNA | Proliferating cell nuclear antigen |
References
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to physiology and disease in women and men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Zhao, Y.; Agarwal, V.R.; Michael, M.D.; Bulun, S.E.; Hinshelwood, M.M.; Graham-Lorence, S.; Sun, T.; Fisher, C.R.; Qin, K.; et al. Aromatase expression in health and disease. Recent Prog. Horm. Res. 1997, 52, 185–213. [Google Scholar] [PubMed]
- Carson-Jurica, M.A.; Schrader, W.T.; O’Malley, B.W. Steroid receptor family: Structure and functions. Endocr. Rev. 1990, 11, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Cheskis, B.J.; Greger, J.G.; Nagpal, S.; Freedman, L.P. Signaling by estrogens. J. Cell. Physiol. 2007, 213, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Sar, M.; Lubahn, D.B.; French, F.S.; Wilson, E.M. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology 1990, 127, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Zubeldia-Brenner, L.; Roselli, C.E.; Recabarren, S.E.; Gonzalez Deniselle, M.C.; Lara, H.E. Developmental and functional effects of steroid hormones on the neuroendocrine axis and spinal cord. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase—A brief overview. Annu. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Burks, D.J.; Vazquez, G.; Rubio, M.; Hernandez, E.; Bodego, P.; Vazquez, R. Expression of aromatase p450 is increased in spontaneous prolactinomas of aged rats. Pituitary 2002, 5, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.H.; Mezzomo, L.C.; Ferreira, N.P.; Roehe, A.V.; Kohek, M.B.; Oliveira Mda, C. Aromatase p450 expression in human pituitary adenomas. Neuropathology 2015, 35, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Morale, M.C.; L’Episcopo, F.; Tirolo, C.; Giaquinta, G.; Caniglia, S.; Testa, N.; Arcieri, P.; Serra, P.A.; Lupo, G.; Alberghina, M.; et al. Loss of aromatase cytochrome p450 function as a risk factor for Parkinson’s disease? Brain Res. Rev. 2008, 57, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2017, 168, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Selek, A.; Cetinarslan, B.; Gurbuz, Y.; Tarkun, I.; Canturk, Z.; Cabuk, B. Aromatase enzyme expression in acromegaly and its possible relationship with disease prognosis. Endocrine 2015, 49, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Hickey, G.J.; Krasnow, J.S.; Beattie, W.G.; Richards, J.S. Aromatase cytochrome p450 in rat ovarian granulosa cells before and after luteinization: Adenosine 3′,5′-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cdna and 5′ genomic DNA. Mol. Endocrinol. 1990, 4, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Barney, M.L.; Patil, J.G.; Gunasekera, R.M.; Carter, C.G. Distinct cytochrome p450 aromatase isoforms in the common carp (Cyprinus carpio): Sexual dimorphism and onset of ontogenic expression. Gen. Comp. Endocrinol. 2008, 156, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of pituitary adenomas: A systematic review. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Rixhon, M.; Adam, C.; Dempegioti, A.; Tichomirowa, M.A.; Beckers, A. High prevalence of pituitary adenomas: A cross-sectional study in the province of liege, belgium. J. Clin. Endocrinol. Metab. 2006, 91, 4769–4775. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Ezzat, S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr. Rev. 1998, 19, 798–827. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Lightman, S. Molecular defects in the pathogenesis of pituitary tumours. Front. Neuroendocrinol. 2003, 24, 94–127. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Z. Relationship between expression of vascular endothelial growth factor and the proliferation of prolactinomas. Clin. Neurol. Neurosurg. 2017, 153, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ragel, B.T.; Couldwell, W.T. Pituitary carcinoma: A review of the literature. Neurosurg. Focus 2004, 16, E7. [Google Scholar] [CrossRef] [PubMed]
- Gillam, M.P.; Molitch, M.E.; Lombardi, G.; Colao, A. Advances in the treatment of prolactinomas. Endocr. Rev. 2006, 27, 485–534. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, J.; Scotton, T.C.; Kang, K.; Zada, G.; Carmichael, J.D. Gene expression in prolactinomas: A systematic review. Pituitary 2016, 19, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Mindermann, T.; Wilson, C.B. Age-related and gender-related occurrence of pituitary adenomas. Clin. Endocrinol. 1994, 41, 359–364. [Google Scholar] [CrossRef]
- Sarkar, D.K. Genesis of prolactinomas: Studies using estrogen-treated animals. Front. Horm. Res. 2006, 35, 32–49. [Google Scholar] [PubMed]
- Saeger, W.; Ludecke, B.; Ludecke, D.K. Clinical tumor growth and comparison with proliferation markers in non-functioning (inactive) pituitary adenomas. Exp. Clin. Endocrinol. Diabetes 2008, 116, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.P.; Fernando, M.; Melmed, S. Functional role of estrogen in pituitary tumor pathogenesis. J. Clin. Investig. 2002, 109, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Faglia, G.; Spada, A. Genesis of pituitary adenomas: State of the art. J. Neuro Oncol. 2001, 54, 95–110. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Jin, L.; Fields, K.; Kulig, E. Effects of estrogens on pituitary cell and pituitary tumor growth. Pathol. Res. Pract. 1991, 187, 584–586. [Google Scholar] [CrossRef]
- Carretero, J.; Blanco, E.J.; Carretero, M.; Carretero-Hernandez, M.; Garcia-Barrado, M.J.; Iglesias-Osma, M.C.; Burks, D.J.; Font de Mora, J. The expression of aib1 correlates with cellular proliferation in human prolactinomas. Ann. Anat. 2013, 195, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Vazquez, G.; Blanco, E.; Rubio, M.; Santos, M.; Martin-Clavijo, A.; Torres, J.L.; Vazquez, R. Immunohistochemical evidence of the presence of aromatase p450 in the rat hypophysis. Cell Tissue Res. 1999, 295, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.A.; Zhou, L.; Vierk, R.; Brandt, N.; Keller, A.; Gee, C.E.; Schafer, M.K.; Rune, G.M. Sex-dependent regulation of aromatase-mediated synaptic plasticity in the basolateral amygdala. J. Neurosci. 2017, 37, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Biegon, A. In vivo visualization of aromatase in animals and humans. Front. Neuroendocrinol. 2016, 40, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, C.D.; Cabrera Zapata, L.E.; Arevalo, M.A.; Garcia-Segura, L.M.; Cambiasso, M.J. Regulation of aromatase expression in the anterior amygdala of the developing mouse brain depends on ERβ and sex chromosome complement. Sci. Rep. 2017, 7, 5320. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, G.; Corvaisier, S.; Kottler, M.L. Aromatase gene expression and regulation in the female rat pituitary. Ann. N. Y. Acad. Sci. 2006, 1070, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, P.; Oral, G.; Sayitoglu, M.; Erensoy, N.; Senel, B.; Gazioglu, N.; Sav, A.; Cetin, G.; Ozbek, U. Aromatase cytochrome p450 enzyme expression in human pituitary. Pituitary 2008, 11, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Nocillado, J.N.; Elizur, A.; Avitan, A.; Carrick, F.; Levavi-Sivan, B. Cytochrome p450 aromatase in grey mullet: Cdna and promoter isolation; brain, pituitary and ovarian expression during puberty. Mol. Cell. Endocrinol. 2007, 263, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Boyar, R.M.; Perlow, M.; Kapen, S.; Lefkowitz, G.; Weitzman, E.; Hellman, L. The effect of clomiphene citrate on the 24-hour lh secretory pattern in normal men. J. Clin. Endocrinol. Metab. 1973, 36, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Vazquez, G.; Rubio, M.; Blanco, E.; Juanes, J.A.; Perez, E.; Burks, D.; Vazquez, R. Postnatal differentiation of the immunohistochemical expression of aromatase p450 in the rat pituitary gland. Histol. Histopathol. 2003, 18, 419–423. [Google Scholar] [PubMed]
- Carretero, J.; Lopez, F.; Catalano-Iniesta, L.; Sanchez-Robledo, V.; Garcia-Barrado, M.J.; Iglesias-Osma, M.C.; Carretero-Hernandez, M.; Blanco, E.J.; Burks, D.J. Pituitary aromatase p450 may be involved in maintenance of the population of luteinizing hormone-positive pituitary cells in mice. Cells Tissues Organs 2016, 201, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Garcia Barrado, M.J.; Blanco, E.J.; Carretero Hernandez, M.; Iglesias Osma, M.C.; Carretero, M.; Herrero, J.J.; Burks, D.J.; Carretero, J. Local transformations of androgens into estradiol by aromatase p450 is involved in the regulation of prolactin and the proliferation of pituitary prolactin-positive cells. PLoS ONE 2014, 9, e101403. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Blanco, E.J.; Iglesias-Osma, M.C.; Carretero-Hernandez, M.; Carretero, M.; Vazquez, T.; Alvarez, P.; Garcia-Barrado, M.J. Morphological evidence of the relevance of aromatase p450 in autoparacrine regulation and pathology of the hypophysis. Eur. J. Anat. 2015, 19, 229–247. [Google Scholar]
- Barraclough, C.A.; Camp, P.; Weiland, N.; Akabori, A. Stimulatory versus inhibitory effects of progesterone on estrogen-induced phasic lh and prolactin secretion correlated with estrogen nuclear and progestin cytosol receptor concentrations in brain and pituitary gland. Neuroendocrinology 1986, 42, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V.; Meunier, H.; Veilleux, R.; Labrie, F. Direct effects of sex steroids on prolactin release at the anterior pituitary level: Interactions with dopamine, thyrotropin-releasing hormone, and isobutylmethylxanthine. Endocrinology 1982, 111, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Kuan, S.I.; MacLeod, R.M. Effect of 17 β-estradiol on phosphoinositide metabolism and prolactin secretion in anterior pituitary cells. Neuroendocrinology 1989, 50, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Maurer, R.A. Estradiol regulates the transcription of the prolactin gene. J. Biol. Chem. 1982, 257, 2133–2136. [Google Scholar] [PubMed]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef] [PubMed]
- Friend, K.E.; Chiou, Y.K.; Lopes, M.B.; Laws, E.R., Jr.; Hughes, K.M.; Shupnik, M.A. Estrogen receptor expression in human pituitary: Correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J. Clin. Endocrinol. Metab. 1994, 78, 1497–1504. [Google Scholar] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [PubMed]
- Carrillo, A.J.; Sharp, Z.D.; DePaolo, L.V. Correlation of rat pituitary prolactin messenger ribonucleic acid and hormone content with serum levels during the estrogen-induced surge. Endocrinology 1987, 121, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Shull, J.D.; Gorski, J. Estrogen regulates the transcription of the rat prolactin gene in vivo through at least two independent mechanisms. Endocrinology 1985, 116, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Kansra, S.; Yamagata, S.; Sneade, L.; Foster, L.; Ben-Jonathan, N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol. Cell. Endocrinol. 2005, 239, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Angoso, M.; Rubio, M.; Blanco, E.J.; Sierra, E.; Herrero, J.J.; Perez, E.; Burks, D.J. In vitro immunoblockade of vip inhibits the proliferation of pituitary prolactin cells. Anat. Embryol. 2006, 211, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Goluboff, L.G.; Ezrin, C. Effect of pregnancy on the somatotroph and the prolactin cell of the human adenohypophysis. J. Clin. Endocrinol. Metab. 1969, 29, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, B.W.; Sano, T.; Kovacs, K.T.; Young, W.F., Jr.; Ryan, N.; Randall, R.V. The pituitary gland in pregnancy: A clinicopathologic and immunohistochemical study of 69 cases. Mayo Clin. Proc. 1990, 65, 461–474. [Google Scholar] [CrossRef]
- Carretero, J.; Sanchez, F.; Blanco, E.; Montero, M.; Riesco, J.M.; Gonzalez, R.; Vazquez, R. Estrogenic modulation of met-enkephalin-induced prolactin secretion in rats. Anat. Embryol. 1991, 183, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Sanchez, F.; Blanco, E.; Riesco, J.M.; Sanchez-Franco, F.; Vazquez, R. Morphofunctional study of mammotropic cells following intraventricular administration of met-enkephalin. Anat. Embryol. 1989, 179, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kineman, R.D.; Faught, W.J.; Frawley, L.S. Steroids can modulate transdifferentiation of prolactin and growth hormone cells in bovine pituitary cultures. Endocrinology 1992, 130, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Osma, M.C.; Blanco, E.J.; Carretero-Hernandez, M.; Garcia-Barrado, M.J.; Catalano-Iniesta, L.; Carretero, M.; Sanchez-Robledo, V.; Carretero, J. Endothelial immunocytochemical expression of pituitary IL-1β and its relation to ACTH-positive cells is regulated by corticosterone in the male rat. Cytokine 2017, 99, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.M.; Neill, J.D. Dopamine levels in hypophysial stalk blood in the rat are sufficient to inhibit prolactin secretion in vivo. Endocrinology 1978, 102, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Saiardi, A.; Bozzi, Y.; Baik, J.H.; Borrelli, E. Antiproliferative role of dopamine: Loss of d2 receptors causes hormonal dysfunction and pituitary hyperplasia. Neuron 1997, 19, 115–126. [Google Scholar] [CrossRef]
- Bowers, C.Y.; Friesen, H.G.; Hwang, P.; Guyda, H.J.; Folkers, K. Prolactin and thyrotropin release in man by synthetic pyroglutamyl-histidyl-prolinamide. Biochem. Biophys. Res. Commun. 1971, 45, 1033–1041. [Google Scholar] [CrossRef]
- Jacobi, J.; Lloyd, H.M.; Meares, J.D. Onset of oestrogen-induced prolactin secretion and DNA synthesis by the rat pituitary gland. J. Endocrinol. 1977, 72, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Mitchner, N.A.; Garlick, C.; Steinmetz, R.W.; Ben-Jonathan, N. Differential regulation and action of estrogen receptors α and β in gh3 cells. Endocrinology 1999, 140, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.S.; Norfleet, A.M.; Pappas, T.C.; Gametchu, B. Rapid actions of estrogens in gh3/b6 pituitary tumor cells via a plasma membrane version of estrogen receptor-α. Steroids 1999, 64, 5–13. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Afratis, N.; Smirlaki, G.; Nikitovic, D.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers: Focus on the role and impact of proteoglycans. Matrix Biol. 2014, 35, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, A.; Cooper, O. Role of tyrosine kinase inhibitors in the treatment of pituitary tumours: From bench to bedside. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kano, M.; Araki, T.; Cooper, O.; Fukuoka, H.; Tone, Y.; Tone, M.; Melmed, S. Erbb receptor-driven prolactinomas respond to targeted lapatinib treatment in female transgenic mice. Endocrinology 2015, 156, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Flageng, M.H.; Larionov, A.; Geisler, J.; Knappskog, S.; Prestvik, W.S.; Bjorkoy, G.; Lilleng, P.K.; Dixon, J.M.; Miller, W.R.; Lonning, P.E.; et al. Treatment with aromatase inhibitors stimulates the expression of epidermal growth factor receptor-1 and neuregulin 1 in ER positive/HER-2/neu non-amplified primary breast cancers. J. Steroid Biochem. Mol. Biol. 2017, 165, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.H.; Park, J.Y.; Rhim, C.C.; Kim, J.H.; Park, Y.; Kim, K.R.; Nam, J.H. Investigation of new therapeutic targets in undifferentiated endometrial sarcoma. Gynecol. Obstet. Investig. 2017, 82, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Okuda, K.; Yano, M.; Oda, R.; Sakane, T.; Kawano, O.; Haneda, H.; Moriyama, S.; Nakanishi, M.; Nakanishi, R. Exon 7 splicing variant of estrogen receptor α is associated with pathological invasiveness in smoking-independent lung adenocarcinoma. Oncol. Lett. 2017, 14, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Cocciadiferro, L.; Miceli, V.; Granata, O.M.; Carruba, G. Merlin, the product of nf2 gene, is associated with aromatase expression and estrogen formation in human liver tissues and liver cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 172, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barrado, M.J.; Blanco, E.J.; Catalano-Iniesta, L.; Sanchez-Robledo, V.; Iglesias-Osma, M.C.; Carretero-Hernandez, M.; Rodriguez-Cobos, J.; Burks, D.J.; Carretero, J. Relevance of pituitary aromatase and estradiol on the maintenance of the population of prolactin-positive cells in male mice. Steroids 2016, 111, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; McArthur, S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson’s disease: A contribution to sex-specific neuroprotection by estrogens. Horm. Behav. 2010, 57, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.H.; Meyer, R.K. Mechanism of anterior pituitary tumor induction by estrogen. Anat. Rec. 1956, 125, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.W.; Riskind, P.N.; Hedley-Whyte, E.T. Vasoactive intestinal peptide in the human pituitary gland and adenomas. An immunocytochemical study. Am. J. Pathol. 1989, 135, 329–338. [Google Scholar] [PubMed]
- Phelps, C.J.; Hymer, W.C. Effects of bromocriptine on prolactin cellular hypertrophy, proliferation and secretory activity in diethylstilbestrol-induced pituitary tumors. Mol. Cell. Endocrinol. 1988, 58, 137–148. [Google Scholar] [CrossRef]
- De Castro, F.; Moron, F.J.; Montoro, L.; Galan, J.J.; Real, L.M.; Ruiz, A. Re: Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J. Natl. Cancer Inst. 2005, 97, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Artigalas, O.; Vanni, T.; Hutz, M.H.; Ashton-Prolla, P.; Schwartz, I.V. Influence of cyp19a1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: A systematic review and meta-analysis. BMC Med. 2015, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Akinci, H.; Kapucu, A.; Dar, K.A.; Celik, O.; Tutunculer, B.; Sirin, G.; Oz, B.; Gazioglu, N.; Ince, H.; Aliustaoglu, S.; et al. Aromatase cytochrome p450 enzyme expression in prolactinomas and its relationship to tumor behavior. Pituitary 2013, 16, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Hosseinpanah, F.; Shirazian, N. Achievement of fertility in an infertile man with resistant macroprolactinoma using high-dose bromocriptine and a combination of human chorionic gonadotropin and an aromatase inhibitor. Endocr. Pract. 2010, 16, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Management of medically refractory prolactinoma. J. Neuro Oncol. 2014, 117, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Pharmacologic resistance in prolactinoma patients. Pituitary 2005, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Gillam, M.P.; Middler, S.; Freed, D.J.; Molitch, M.E. The novel use of very high doses of cabergoline and a combination of testosterone and an aromatase inhibitor in the treatment of a giant prolactinoma. J. Clin. Endocrinol. Metab. 2002, 87, 4447–4451. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Perez, E.; Watanabe, Y.G. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990, 261, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Okazaki, K.; Kawashima, S. Mitotic activity of prolactin cells in the pituitary glands of male and female rats of different ages. Cell Tissue Res. 1984, 235, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Drewett, N.; Jacobi, J.M.; Willgoss, D.A.; Lloyd, H.M. Apoptosis in the anterior pituitary gland of the rat: Studies with estrogen and bromocriptine. Neuroendocrinology 1993, 57, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G. Predictive markers of pituitary adenoma behavior. Neuroendocrinology 2006, 83, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Pickering, R.M.; Gawne-Cain, M.L.; King, S.; Lees, P.D.; Ellison, D.W. Indices of apoptosis and proliferation as potential prognostic markers in non-functioning pituitary adenomas. Clin. Neuropathol. 2004, 23, 8–15. [Google Scholar] [PubMed]
- Nakabayashi, H.; Sunada, I.; Hara, M. Immunohistochemical analyses of cell cycle-related proteins, apoptosis, and proliferation in pituitary adenomas. J. Histochem. Cytochem. 2001, 49, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Seilicovich, A. Cell life and death in the anterior pituitary gland: Role of oestrogens. J. Neuroendocrinol. 2010, 22, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, V.; Magri, M.L.; Zarate, S.; Jaita, G.; Eijo, G.; Radl, D.; Ferraris, J.; Pisera, D.; Seilicovich, A. Estradiol increases the bax/bcl-2 ratio and induces apoptosis in the anterior pituitary gland. Neuroendocrinology 2009, 90, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Magri, M.L.; Gottardo, M.F.; Zarate, S.; Eijo, G.; Ferraris, J.; Jaita, G.; Ayala, M.M.; Candolfi, M.; Pisera, D.; Seilicovich, A. Opposite effects of dihydrotestosterone and estradiol on apoptosis in the anterior pituitary gland from male rats. Endocrine 2016, 51, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Amara, J.F.; van Itallie, C.; Dannies, P.S. Regulation of prolactin production and cell growth by estradiol: Difference in sensitivity to estradiol occurs at level of messenger ribonucleic acid accumulation. Endocrinology 1987, 120, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Rubio, M.; Navarro, N.; Prieto, P.; Vazquez, R.J.; Sanchez, F.; Vazquez, R. In vitro modifications in the proliferation rate of prolactin cells are accompanied by nuclear morphometric variations. Histol. Histopathol. 1995, 10, 135–139. [Google Scholar] [PubMed]
- Manoranjan, B.; Salehi, F.; Scheithauer, B.W.; Rotondo, F.; Kovacs, K.; Cusimano, M.D. Estrogen receptors α and β immunohistochemical expression: Clinicopathological correlations in pituitary adenomas. Anticancer Res. 2010, 30, 2897–2904. [Google Scholar] [PubMed]
- Shupnik, M.A.; Pitt, L.K.; Soh, A.Y.; Anderson, A.; Lopes, M.B.; Laws, E.R., Jr. Selective expression of estrogen receptor α and β isoforms in human pituitary tumors. J. Clin. Endocrinol. Metab. 1998, 83, 3965–3972. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, H.M.; Comunoglu, N.; Keskin, F.E.; Oz, B.; Haliloglu, O.A.; Tanriover, N.; Gazioglu, N.; Kadioglu, P. Locally produced estrogen through aromatization might enhance tissue expression of pituitary tumor transforming gene and fibroblast growth factor 2 in growth hormone-secreting adenomas. Endocrine 2016, 52, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Mantovani, G.; Lania, A. Pathogenesis of prolactinomas. Pituitary 2005, 8, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Horwitz, G.A.; Heaney, A.P.; Nakashima, M.; Prezant, T.R.; Bronstein, M.D.; Melmed, S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J. Clin. Endocrinol. Metab. 1999, 84, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, M. Endocrine/paracrine control of pituitary cell proliferation and its involvement in pituitary tumorigenesis. Pituitary 1999, 1, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.K.; Wu, H. The role of aib1 in breast cancer. Oncol. Lett. 2012, 4, 588–594. [Google Scholar] [PubMed]
- Edwards, D.P. Regulation of signal transduction pathways by estrogen and progesterone. Annu. Rev. Physiol. 2005, 67, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Bardou, V.; Hopp, T.A.; Chamness, G.C.; Hilsenbeck, S.G.; Fuqua, S.A.; Wong, J.; Allred, D.C.; Clark, G.M.; Schiff, R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. 2003, 95, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Bouras, T.; Southey, M.C.; Venter, D.J. Overexpression of the steroid receptor coactivator aib1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and her2/neu. Cancer Res. 2001, 61, 903–907. [Google Scholar] [PubMed]
- Wang, M.; Zhao, F.; Li, S.; Chang, A.K.; Jia, Z.; Chen, Y.; Xu, F.; Pan, H.; Wu, H. AIB1 cooperates with ERα to promote epithelial mesenchymal transition in breast cancer through SNAI1 activation. PLoS ONE 2013, 8, e65556. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arzayus, M.I.; Font de Mora, J.; Yuan, J.; Vazquez, F.; Bronson, R.; Rue, M.; Sellers, W.R.; Brown, M. High tumor incidence and activation of the pi3k/akt pathway in transgenic mice define aib1 as an oncogene. Cancer Cell. 2004, 6, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Avivar, A.; Garcia-Macias, M.C.; Ascaso, E.; Herrera, G.; O’Connor, J.E.; Font de Mora, J. Moderate overexpression of aib1 triggers pre-neoplastic changes in mammary epithelium. FEBS Lett. 2006, 580, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Arai, S.; Nishihara, T.; Nishikawa, J. Aib1 promotes DNA replication by jnk repression and akt activation during cellular stress. J. Biochem. 2006, 140, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, D.; Chalifour, L.E.; Alaoui, J.; Ma, H.T.H.; Alaoui-Jamali, M.A.; Alaoui-Jamali, M.A.; Huynh, H.T.; Huynh, H.T. Steroid-sensitive gene-1 is an androgen-regulated gene expressed in prostatic smooth muscle cells in vivo. J. Mol. Endocrinol. 2001, 26, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Planas-Silva, M.D.; Shang, Y.; Donaher, J.L.; Brown, M.; Weinberg, R.A. Aib1 enhances estrogen-dependent induction of cyclin d1 expression. Cancer Res. 2001, 61, 3858–3862. [Google Scholar] [PubMed]
- Zhou, H.J.; Yan, J.; Luo, W.; Ayala, G.; Lin, S.H.; Erdem, H.; Ittmann, M.; Tsai, S.Y.; Tsai, M.J. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005, 65, 7976–7983. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.Q.; Liao, L.; Zhang, H.; Lee, A.V.; O’Malley, B.W.; Xu, J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004, 64, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.; Avivar, A.; Garcia-Macias, M.C.; Font de Mora, J. Phosphoinositide 3-kinase/AKT signaling can promote AIB1 stability independently of gsk3 phosphorylation. Cancer Res. 2008, 68, 5450–5459. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Oh, A.S.; Bowden, E.T.; Lahusen, T.; Lorick, K.L.; Weissman, A.M.; Schlegel, R.; Wellstein, A.; Riegel, A.T. E6ap mediates regulated proteasomal degradation of the nuclear receptor coactivator amplified in breast cancer 1 in immortalized cells. Cancer Res. 2006, 66, 8680–8686. [Google Scholar] [CrossRef] [PubMed]
- Caccavelli, L.; Morange-Ramos, I.; Kordon, C.; Jaquet, P.; Enjalbert, A. Alteration of g α subunits mrna levels in bromocriptine resistant prolactinomas. J. Neuroendocrinol. 1996, 8, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. P53 function and dysfunction. Cell 1992, 70, 523–526. [Google Scholar] [CrossRef]
- Chang, F.; Syrjanen, S.; Kurvinen, K.; Syrjanen, K. The p53 tumor suppressor gene as a common cellular target in human carcinogenesis. Am. J. Gastroenterol. 1993, 88, 174–186. [Google Scholar] [PubMed]
- Bailey, S.T.; Shin, H.; Westerling, T.; Liu, X.S.; Brown, M. Estrogen receptor prevents p53-dependent apoptosis in breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 18060–18065. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Katzenellenbogen, B.S. Estrogen receptor-β modulation of the erα-p53 loop regulating gene expression, proliferation, and apoptosis in breast cancer. Horm. Cancer 2017, 8, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, A.; Konduri, S.D.; Liu, W.; Bansal, S.; Li, F.; Das, G.M. Estrogen receptor α inhibits p53-mediated transcriptional repression: Implications for the regulation of apoptosis. Cancer Res. 2007, 67, 7746–7755. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Simpson, E.R.; Brown, K.A. P53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 2015, 75, 5001–5007. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Simpson, E.R.; Brown, K.A. Inflammation, dysregulated metabolism and aromatase in obesity and breast cancer. Curr. Opin. Pharmacol. 2016, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.H.; Magalhaes, A.V.; Mello, P.A.; Schmitt, F.C.; Casulari, L.A. Expression of p53, Ki-67 and c-erb B2 in growth hormone-and/or prolactin-secreting pituitary adenomas. Arq Neuropsiquiatr. 2006, 64, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, F.; Jiang, L.; Hu, G.; Sun, W.; Zhang, C.; Ding, X. Inhibition of skp2 sensitizes bromocriptine-induced apoptosis in human prolactinoma cells. Cancer Res. Treat. 2017, 49, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Marroni, C.P.; Pizarro, C.B.; Pereira-Lima, J.F.; Barbosa-Coutinho, L.M.; Ferreira, N.P. Expression of p53 protein in pituitary adenomas. Braz. J. Med. Biol. Res. 2002, 35, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; East, H.E.; French, S.E.; Melcescu, E.; Hamilton, R.D.; Nicholas, W.C.; Fratkin, J.F.; Parent, A.D.; Luzardo, G.; Koch, C.A. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones 2012, 11, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R. Murine models of neoplasia: Functional analysis of the tumour suppressor genes rb-1 and p53. Cancer Metastasis Rev. 1995, 14, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Woloschak, M.; Yu, A.; Xiao, J. Molecular and cellular responses to DNA damage in a murine pituitary adenoma cell line. Mol. Cell. Endocrinol. 1996, 119, 61–68. [Google Scholar] [CrossRef]
- Aoki, M.P.; Aoki, A.; Maldonado, C.A. Sexual dimorphism of apoptosis in lactotrophs induced by bromocryptine. Histochem. Cell. Biol. 2001, 116, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Tamaki, N.; Kokunai, T.; Yasuo, K.; Yonezawa, K. Bromocriptine-induced apoptosis in pituitary adenoma cells: Relationship to p53 and bcl-2 expression. J. Clin. Neurosci. 1999, 6, 326–331. [Google Scholar] [CrossRef]
- Sav, A.; Rotondo, F.; Syro, L.V.; Scheithauer, B.W.; Kovacs, K. Biomarkers of pituitary neoplasms. Anticancer Res. 2012, 32, 4639–4654. [Google Scholar] [PubMed]
- Polyak, K.; Kato, J.Y.; Solomon, M.J.; Sherr, C.J.; Massague, J.; Roberts, J.M.; Koff, A. P27kip1, a cyclin-cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994, 8, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.; Canda, M.S.; Ulukus, C.; Guray, M.; Erbayraktar, S. Expression of bcl-2, bax and p53 proteins in pituitary adenomas: An immunohistochemical study. Tumori 2003, 89, 54–59. [Google Scholar] [PubMed]
- Facchetti, M.; Uberti, D.; Memo, M.; Missale, C. Nerve growth factor restores p53 function in pituitary tumor cell lines via trka-mediated activation of phosphatidylinositol 3-kinase. Mol. Endocrinol. 2004, 18, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Pernicone, P.J.; Scheithauer, B.W.; Sebo, T.J.; Kovacs, K.T.; Horvath, E.; Young, W.F., Jr.; Lloyd, R.V.; Davis, D.H.; Guthrie, B.L.; Schoene, W.C. Pituitary carcinoma: A clinicopathologic study of 15 cases. Cancer 1997, 79, 804–812. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, H.J.; Kim, J.W.; Lee, J.W. Steroid receptor coactivator-1 and its family members differentially regulate transactivation by the tumor suppressor protein p53. Mol. Endocrinol. 1999, 13, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Sham, J.S.; Zeng, W.F.; Lin, H.L.; Bi, J.; Che, L.H.; Hu, L.; Zeng, Y.X.; Guan, X.Y. Correlation of aib1 overexpression with advanced clinical stage of human colorectal carcinoma. Hum. Pathol. 2005, 36, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Hunter, T. P27, a novel inhibitor of g1 cyclin-CDK protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Musat, M.; Korbonits, M.; Pyle, M.; Gueorguiev, M.; Kola, B.; Morris, D.G.; Powell, M.; Dumitrache, C.; Poiana, C.; Grossman, A.B. The expression of the f-box protein skp2 is negatively associated with p27 expression in human pituitary tumors. Pituitary 2002, 5, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tomono, Y.; Nose, T. Expression of p27kip1 and ki-67 in pituitary adenomas: An investigation of marker of adenoma invasiveness. Acta Neurochir. 1999, 141, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Erickson, L.A.; Jin, L.; Kulig, E.; Qian, X.; Cheville, J.C.; Scheithauer, B.W. P27kip1: A multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am. J. Pathol. 1999, 154, 313–323. [Google Scholar] [CrossRef]
- Ikeda, H.; Yoshimoto, T.; Shida, N. Molecular analysis of p21 and p27 genes in human pituitary adenomas. Br. J. Cancer 1997, 76, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.S.; Camargo, R.C.; Saggioro, F.P.; Neder, L.; Machado, H.R.; Moreira, A.C.; de Castro, M. P27/CDKN1B translational regulators in pituitary tumorigenesis. Horm. Metab. Res. 2016, 48, 840–846. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Barrado, M.J.; Blanco, E.J.; Iglesias-Osma, M.C.; Carretero-Hernández, M.; Catalano-Iniesta, L.; Sanchez-Robledo, V.; Carretero, M.; Herrero, J.J.; Carrero, S.; Carretero, J. Relation among Aromatase P450 and Tumoral Growth in Human Prolactinomas. Int. J. Mol. Sci. 2017, 18, 2299. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18112299
García-Barrado MJ, Blanco EJ, Iglesias-Osma MC, Carretero-Hernández M, Catalano-Iniesta L, Sanchez-Robledo V, Carretero M, Herrero JJ, Carrero S, Carretero J. Relation among Aromatase P450 and Tumoral Growth in Human Prolactinomas. International Journal of Molecular Sciences. 2017; 18(11):2299. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18112299
Chicago/Turabian StyleGarcía-Barrado, María José, Enrique J. Blanco, María Carmen Iglesias-Osma, Marta Carretero-Hernández, Leonardo Catalano-Iniesta, Virginia Sanchez-Robledo, Manuel Carretero, Julio Joaquín Herrero, Sixto Carrero, and José Carretero. 2017. "Relation among Aromatase P450 and Tumoral Growth in Human Prolactinomas" International Journal of Molecular Sciences 18, no. 11: 2299. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18112299