Nitroxides as Antioxidants and Anticancer Drugs
Abstract
:1. Introduction
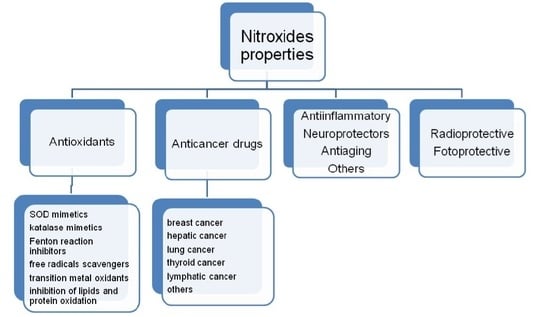
2. Properties of Nitroxides
3. Nitroxides in Cancer Therapy
3.1. Breast Cancer
3.2. Hepatic Cancer
3.3. Lung Cancer
3.4. Thyroid Cancer
3.5. Ovarian Cancer
3.6. Lymphatic Cancer
3.7. Other Cancers
3.8. Nitroxides and Cis-platin Toxicity
3.9. Nitroxides and Doxorubicin Toxicity
4. Nitroxides in Aging and Diseases
5. Concluding Remarks
Acknowledgments
Conflicts of Interest
Abbreviations
| BMK1 | Big mitogen-activated protein kinase 1 |
| CAT | catalase |
| CK-MB | creatine kinase-MB |
| ERK1/2 | extracellular signal-regulated kinase 1 and 2, fluorouracil (5-FU) |
| GSH | glutathione (reduced form) |
| JNK | c-Jun N-terminal kinase, |
| LDH | lactic dehydrogenase |
| MMP-2 | matrix metalloproteinase-2 |
| NADH | (nicotinamide adenine dinucleotide, reduced form) |
| PARP | (poly(ADP-ribose) polymerase) |
| SOD | superoxide dismutase |
References
- Tabaczar, S.; Talar, M.; Gwozdzinski, K. Nitroxides as antioxidants—Possibilities of their application in chemoprevention and radioprotection. Postepy Hig. Med. Doswiadczalnej 2011, 65, 46–54. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Rimoldi, V.; Supino, R.; Favini, E.; Monti, E. The nitroxide tempol induces oxidative stress, p21(WAF1/CIP1), and cell death in HL60 cells. Free Radic. Biol. Med. 2000, 29, 633–641. [Google Scholar] [CrossRef]
- Samuni, A.; Krishna, C.M.; Riesz, P.; Fikelstein, E.; Russo, A. Superoxide reaction with nitroxide spin-adducts. Free Radic. Biol. Med. 1989, 6, 141–148. [Google Scholar] [CrossRef]
- Voest, E.E.; van Faassen, E.; Marx, J.J. An electron paramagnetic resonance study of the antioxidant properties of the nitroxide free radical TEMPO. Free Radic. Biol. Med. 1993, 15, 589–595. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Lucchi, S.; Caserini, C.; Supino, R.; Oliva, C.; Monti, E. Antiproliferative effect of piperidine nitroxide tempol on neoplastic and non-neoplastic mammalian cell lines. Free Radic. Biol. Med. 1998, 24, 913–923. [Google Scholar] [CrossRef]
- Goralska, M.; Holley, B.; Mcgahan, M.C. The effects of tempol on ferritin synthesis and Fe metabolism in lens epithelial cells. Biochim. Biophys. Acta Mol. Cell Res. 2000, 1497, 51–60. [Google Scholar] [CrossRef]
- Gueven, N.; Luff, J.; Peng, C.; Hosokawa, K.; Bottle, S.E.; Lavin, M.F. Dramatic extension of tumor latency and correction of neurobehavioral phenotype in Atm-mutant mice with a nitroxide antioxidant. Free Radic. Biol. Med. 2006, 41, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Leker, R.R.; Teichner, A.; Lavie, G.; Shohami, E.; Lamensdorf, I.; Ovadia, H. The nitroxide antioxidant tempol is cerebroprotective against focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol. 2002, 176, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Beigrezaei, S.; Nasri, H. Tempol as an antioxidant; an updated review on current knowledge. Ann. Res. Antioxid. 2017, 2, e01. [Google Scholar]
- Liu, Y.Q.; Ohkoshi, E.; Li, L.H.; Yang, L.; Lee, K.H. Design, synthesis and cytotoxic activity of novel spin-labeled rotenone derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Pinegin, B.V. Effects of the antioxidants Trolox, Tiron and Tempol on neutrophil extracellular trap formation. Immunobiology 2016, 221, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.; Tarpey, M.; Krishna, M.; Mitchell, J.B.; Welch, W.J.; Wilcox, C.S. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R37–R43. [Google Scholar]
- Zhao, B.; Pan, Y.; Wang, Z.; Tan, Y.; Song, X. Intrathecal Administration of Tempol Reduces Chronic Constriction Injury-Induced Neuropathic Pain in Rats by Increasing SOD Activity and Inhibiting NGF Expression. Cell. Mol. Neurobiol. 2016, 36, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Dickey, J.S.; Gonzalez, Y.; Aryal, B.; Mog, S.; Nakamura, A.J.; Redon, C.E.; Baxa, U.; Rosen, E.; Cheng, G.; Zielonka, J.; et al. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngenic breast tumor preclinical model. PLoS ONE 2013, 8, e70575. [Google Scholar] [CrossRef] [PubMed]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Grahame, D.A.; Samuni, A.; Mitchell, J.B.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, N.; Pinnick, H.W. Reduction of Nitroxides to Amines by Sodium Sulfide. J. Org. Chem. 1972, 37, 2050–2051. [Google Scholar] [CrossRef]
- Zakrzewski, J. A reaction of nitroxides with ethyl mercaptane: A mild method for conversion of nitroxides into their corresponding amines. Monatsh. Chem. 1990, 121, 803–808. [Google Scholar] [CrossRef]
- Einhorn, J.; Einhorn, C.; Ratajczak, F.; Durif, A.; Averbuch, M.T.; Pierre, J.L. Synthesis and resolution of chiral analogue of 2,2,6,6-tetrametethylopiperidine and of its corresponding nitroxide. Tetrahedron Lett. 1998, 39, 2565–2568. [Google Scholar] [CrossRef]
- Chateauneuf, J.; Lusztyk, J.; Ingold, K.U. Absolute rate constants for the reactions of some carbon-centered radicals with 2,2,6,6-tetramethylpiperidine-N-oxyl. J. Org. Chem. 1988, 53, 1629–1632. [Google Scholar] [CrossRef]
- Krishna, M.C.; Russo, A.; Mitchell, J.B.; Goldstein, S.; Dafni, H.; Samuni, A. Do nitroxide antioxidants act as scavengers of O2−. or as SOD mimics? J. Biol. Chem. 1996, 271, 26026–26031. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.M.; Degraff, W.; Krishna, M.C.; Mitchell, J.B. Nitroxides as antioxidants: Tempol protects against EO9 cytotoxicity. Mol. Cell. Biochem. 2002, 234–235, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Krishna, C.M.; Mitchell, J.B.; Collins, C.R.; Russo, A. Superoxide reaction with nitroxides. Free Radic. Res. Commun. 1990, 9, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Min, A.; Krishna, C.M.; Mitchell, J.B.; Russo, A. SOD-like activity of 5-membered ring nitroxide spin labels. Adv. Exp. Med. Biol. 1990, 264, 85–92. [Google Scholar] [PubMed]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Superoxide-dependent reduction of nitroxides by thiols. Biochim. Biophys. Acta 1984, 802, 90–98. [Google Scholar] [CrossRef]
- Bujak, S.; Gwozdzinski, K. Nitroxides lead to reduced level of glutathione in red blood cells. In Free Radical and Oxidative Stress: Chemistry and Pathological Implications; Galaris, G., Ed.; Medimond International Proceedings: Bologna, Italy, 2003; pp. 105–108. [Google Scholar]
- Glebska, J.; Skolimowski, J.; Kudzin, Z.; Gwozdzinski, K.; Grzelak, A.; Bartosz, G. Pro-oxidative activity of nitroxides in their reactions with glutathione. Free Radic. Biol. Med. 2003, 35, 310–316. [Google Scholar] [CrossRef]
- Glebska, J.; Gwozdzinski, K. Oxygen-dependent reduction of nitroxides by ascorbic acid and glutathione. EPR investigations. Curr. Top. Biophys. 1998, 22, 75–82. [Google Scholar]
- Czepas, J.; Koceva-Chyla, A.; Gwozdzinski, K.; Jozwiak, Z. Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide. Cell Biol. Toxicol. 2008, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Bartosz, G. Nitroxide reduction in human red blood cells. Curr. Top. Biophys. 1996, 20, 60–65. [Google Scholar]
- Gwozdzinski, K.; Bartosz, G.; Leyko, W. Effect of gamma radiation on the transport of spin-labeled compounds across the erythrocyte membrane. Radiat. Environ. Biophys. 1981, 19, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Bartosz, G.; Leyko, W. Effect of gamma radiation on the transport of electrolyte spin labels across human erytrocyte. Stud. Biophys. 1982, 89, 141–145. [Google Scholar]
- Hyodo, F.; Matsumoto, K.; Matsumoto, A.; Mitchell, J.B.; Krishna, M.C. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006, 66, 9921–9928. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Degraff, W.; Hankovsky, O.; Sar, C.P.; Kalai, T.; Jeko, J.; Russo, A.; Mitchell, J.B. Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J. Med. Chem. 1998, 41, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Samuni, A.; Taira, J.; Goldstein, S.; Mitchell, J.B.; Russo, A. Stimulation by nitroxides of catalase-like activity of hemeproteins. J. Biol. Chem. 1996, 271, 26018–26025. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.M.; Degraff, W.; Krishna, M.C.; Mitchell, J.B. Cellular sites of H2O2-induced damage and their protection by nitroxides. Biochim. Biophys. Acta 2001, 1525, 70–76. [Google Scholar] [CrossRef]
- Wu, Y.J.; Li, W.G.; Zhang, Z.M.; Tian, X. Antioxidative activity of 4-oxy- and 4-hydroxy-nitroxides in tissues and erythrocytes from rats. Zhongguo Yao Li Xue Bao 1997, 18, 150–154. [Google Scholar] [PubMed]
- Dikalov, S.; Grigor’ev, I.A.; Voinov, M.; Bassenge, E. Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: Quantification of extracellular superoxide radicals formation. Biochem. Biophys. Res. Commun. 1998, 248, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Gadjeva, V.; Kuchukova, D.; Tolekova, A.; Tanchev, S. Beneficial effects of spin-labelled nitrosourea on CCNU-induced oxidative stress in rat blood compared with vitamin E. Pharmazie 2005, 60, 530–532. [Google Scholar] [PubMed]
- Nilsson, U.A.; Olsson, L.I.; Carlin, G.; Bylund-Fellenius, A.C. Inhibition of lipid peroxidation by spin labels: Relationships between structure and function. J. Biol. Chem. 1989, 264, 11131–11135. [Google Scholar] [PubMed]
- Schnackenberg, C.G.; Wilcox, C.S. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-isoprostaglandin F2α. Hypertension 1999, 33, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Gao, M.T.; Zheng, R.L. The relationship between structure and antioxidative activity of piperidinenitroxides. J. Pharm. Pharmacol. 2006, 58, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Offer, T.; Samuni, A. Nitroxides inhibit peroxyl radical-mediated DNA scission and enzyme inactivation. Free Radic. Biol. Med. 2002, 32, 872–881. [Google Scholar] [CrossRef]
- Yoshino, F.; Shoji, H.; Lee, M.C. Vascular effects of singlet oxygen (1O2) generated by photo-excitation on adrenergic neurotransmission in isolated rabbit mesenteric vein. Redox Rep. 2002, 7, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.T.; Galatsis, P.; Borosky, S.; Kopec, K.K.; Kumar, V.; Althaus, J.S.; Hall, E.D. 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol. 2000, 13, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mcdonald, M.C.; Mazzon, E.; Filipe, H.M.; Centorrino, T.; Lepore, V.; Terranova, M.L.; Ciccolo, A.; Caputi, A.P.; Thiemermann, C. Beneficial effects of tempol, a membrane-permeable radical scavenger, on the multiple organ failure induced by zymosan in the rat. Crit. Care Med. 2001, 29, 102–111. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (-)Δ9-tetrahydrocannabinol and cannabidiol in N-methyl-d-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Medinas, D.B.; Alves, M.J.; Augusto, O. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic. Biol. Med. 2005, 38, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, O.M.; Maltby, D.A.; Medzihradszky, K.F.; de Montellano, P.R.; Tomer, K.B.; Mason, R.P.; Deterding, L.J. Spin scavenging analysis of myoglobin protein-centered radicals using stable nitroxide radicals: Characterization of oxoammonium cation-induced modifications. Chem. Res. Toxicol. 2009, 22, 1034–1049. [Google Scholar] [CrossRef] [PubMed]
- Charloux, C.; Paul, M.; Loisance, D.; Astier, A. Inhibition of hydroxyl radical production by lactobionate, adenine, and tempol. Free Radic. Biol. Med. 1995, 19, 699–704. [Google Scholar] [CrossRef]
- Samuni, A.; Mitchell, J.B.; Degraff, W.; Krishna, C.M.; Samuni, U.; Russo, A. Nitroxide SOD-mimics: Mode of action. Free Radic. Res. Commun. 1991, 12–13, 187–194. [Google Scholar] [CrossRef]
- Zeltcer, G.; Berenshtein, E.; Samuni, A.; Chevion, M. Nitroxide radicals prevent metal-aggravated reperfusion injury in isolated rat heart. Free Radic. Res. 1997, 27, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Lopez, M.; Zielonka, J.; Hauser, A.D.; Joseph, J.; Mcallister, D.; Rowe, J.J.; Sugg, S.L.; Williams, C.L.; Kalyanaraman, B. Mitochondria-targeted nitroxides exacerbate fluvastatin-mediated cytostatic and cytotoxic effects in breast cancer cells. Cancer. Biol. Ther. 2011, 12, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; McAllister, D.; Hardy, M.; Ouari, O.; Joseph, J.; Dwinell, M.B.; Kalyanaraman, B. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015, 365, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Koceva-Chyla, A.; Czepas, J.; Pieniazek, A.; Piasecka-Zelga, J.; Gwozdzinski, K. Nitroxide pirolin reduces oxidative stress generated by doxorubicin and docetaxel in blood plasma of rats bearing mammary tumor. J. Physiol. Pharmacol. 2012, 63, 153–163. [Google Scholar] [PubMed]
- Tabaczar, S.; Domeradzka, K.; Czepas, J.; Piasecka-Zelga, J.; Stetkiewicz, J.; Gwozdzinski, K.; Koceva-Chyla, A. Anti-tumor potential of nitroxyl derivative Pirolin in the DMBA-induced rat mammary carcinoma model: A comparison with quercetin. Pharmacol. Rep. 2015, 67, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Zhao, X.B.; Wu, D.; Wang, M.J.; Goto, M.; Morris-Natschke, S.L.; Liu, Y.Q.; Wu, X.B.; Song, Z.L.; Zhu, G.X.; Lee, K.H. Design and synthesis of novel spin-labeled camptothecin derivatives as potent cytotoxic agents. Bioorg. Med. Chem. 2014, 22, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.; Redfearn, E.R. The inhibition of mitochondrial reduced NADH oxidation by rotenoids. Biochim. Biophys. Acta 1965, 110, 475–483. [Google Scholar] [CrossRef]
- Deng, Y.T.; Huang, H.C.; Lin, J.K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Dilip, A.; Cheng, G.; Joseph, J.; Kunnimalaiyaan, S.; Kalyanaraman, B.; Kunnimalaiyaan, M.; Gamblin, T.C. Mitochondria-targeted antioxidant and glycolysis inhibition: Synergistic therapy in hepatocellular carcinoma. Anticancer Drugs 2013, 24, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Y.; Zhang, J.; Liang, J.; Zeng, L.; Guo, G. Anticancer effect of tert-butyl-2(4,5-dihydrogen-4,4,5,5-tetramethyl-3-o-1h-imidazole-3-cationic-1-oxyl-2)-pyrrolidine-1-carboxylic ester on human hepatoma HepG2 cell line. Chem. Biol. Interact. 2012, 199, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Bu, W.; Wei, M.Y.; Li, W.W.; Yao, M.N.; Ma, Z.Y.; Lu, C.T.; Li, H.H.; Hu, N.P.; et al. Synthesis of a novel adamantyl nitroxide derivative with potent anti-hepatoma activity in vitro and in vivo. Am. J. Cancer Res. 2016, 6, 1271–1286. [Google Scholar] [PubMed]
- Pettit, G.; Singh, S.B.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendall, D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatinA-4. Experientia 1989, 7, 209–211. [Google Scholar] [CrossRef]
- Ma, M.; Sun, L.; Lou, H.; Ji, M. Synthesis and biological evaluation of Combretastatin A-4 derivatives containing a 3′-O-substituted carbonic ether moiety as potential antitumor agents. Chem. Cent. J. 2013, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Li, X.J.; Zhao, C.Y.; Nan, X.; Tian, J.; Morris-Natschke, S.L.; Zhang, Z.J.; Yang, X.M.; Yang, L.; Li, L.H.; et al. Synthesis and mechanistic studies of novel spin-labeled combretastatin derivatives as potential antineoplastic agents. Bioorg. Med. Chem. 2013, 21, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, W.; Li, C.L.; Liu, J.W.; Miao, L.D.; Han, J.; Lan, M.B. Cytotoxicity of a newly synthesized nitroxide derivtive of 4-ferrocenecarboxyl-2,2,6,6-tetramethylpiperidine-1-oxyl in high metastatic lung tumor cells. Pharmazie 2006, 61, 1028–1033. [Google Scholar] [PubMed]
- Ghoshal, K.; Jacob, S.T. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem. Pharmacol. 1997, 53, 1569–1575. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.W.; Gong, T.; Zhang, Z.R. Synthesis and characterization of insulin-5-Fu conjugate, enabling insulin as multi-drug carrier via dendritic approach. Chin. Chem. Lett. 2007, 18, 247–250. [Google Scholar] [CrossRef]
- Isanbor, C.; O’Hagan, D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluor Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Yang, L.; Wang, M.J.; Zhang, Z.J.; Morris-Natschke, S.L.; Goto, M.; Tian, J.; Liu, Y.Q.; Wang, C.Y.; Tian, X.; Yang, X.M.; et al. Synthesis of novel spin-labeled derivatives of 5-FU as potential antineoplastic agents. Med. Chem. Res. 2014, 23, 3269–3273. [Google Scholar] [CrossRef] [PubMed]
- Starenki, D.; Park, J.I. Mitochondria-targeted nitroxide, MITO-CP, suppresses medullary thyroid carcinoma cell survival in vitro and in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Kuppusamy, M.L.; TazI, M.; Bratasz, A.; Tong, L.; Rivera, B.K.; Kalai, T.; Hideg, K.; et al. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: Differential cytotoxicity in healthy and cancer cells. Free Radic. Biol. Med. 2010, 48, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Monti, E.; Supino, R.; Colleoni, M.; Costa, B.; Ravizza, R.; Gariboldi, M.B. Nitroxide tempol impairs mitochondrial function and induces apoptosis in HL60 cells. J. Cell. Biochem. 2001, 82, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Erker, L.; Barlow, C.; Yakushiji, H.; Larson, D.; Russo, A.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004, 13, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mittelstaedt, R.A.; Guo, L.; Shaddock, J.G.; Heflich, R.H.; Bigger, A.H.; Moore, M.M.; Mei, N. Nitroxide TEMPO: A genotoxic and oxidative stress inducer in cultured cells. Toxicol. In Vitro 2013, 27, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Koceva-Chyla, A.; Kochman, A.; Glebska, J.; Gwozdzinski, K.; Jozwiak, Z.; Metodiewa, D. Tempicol-3, a novel low toxic piperidine-N-oxide stable radical and antioxidant, acts as apoptosis inducer and cell proliferation modifier of Yoshida sarcoma cells in vivo. Anticancer Res. 2000, 20, 4611–4618. [Google Scholar] [PubMed]
- Tabaczar, S.; Czepas, J.; Koceva-Chyla, A.; Kilańczyk, E.; Piasecka-Zelga, J.; Gwoździński, K. The effect of the nitroxide Pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J. Physiol. Pharmacol. 2017, 68, 295–308. [Google Scholar] [PubMed]
- Hong, S.K.; Starenki, D.; Wu, P.K.; Park, J.I. Suppression of B-RafV600E melanoma cell survival by targeting mitochondria using triphenyl-phosphonium-conjugated nitroxide or ubiquinone. Cancer Biol. Ther. 2017, 18, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, S.; Yoshitomi, T.; Sakharkar, M.K.; Nagasaki, Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J. Controll. Release 2015, 209, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Panda, D. Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding. FEBS J. 2007, 274, 4788–4801. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Shreeves, G.; Nathan, P.D.; Gaya, A.; Ganesan, T.S.; Wang, D.; Boxall, J.; Poupard, L.; Chaplin, D.J.; Stratford, M.R.; et al. A Phase Ib trial of CA4P (combretastatin A-4 phosphate), carboplatin, and paclitaxel in patients with advanced cancer. Br. J. Cancer 2010, 102, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; Castro, M.A.; del Corral, J.M.; Feliciano, A.S. Antitumor properties of podophyllotoxin and related compounds. Curr. Pharm. Des. 2000, 6, 1811–1839. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, Y.; Yang, M.G.; Chen, Y.Z. Synthesis and antitumor activity of spin labeled derivatives of podophyllotoxin. Life Sci. 1997, 60, 511–517. [Google Scholar] [CrossRef]
- Kou, L.; Wang, M.J.; Wang, L.T.; Zhao, X.B.; Nan, X.; Yang, L.; Liu, Y.Q.; Morris-Natschke, S.L.; Lee, K.H. Toward synthesis of third-generation spin-labeled podophyllotoxin derivatives using isocyanide multicomponent reactions. Eur. J. Med. Chem. 2014, 75, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pinson, A.; Samuni, A. Both hydroxylamine and nitroxide protects cardiomiocytes from oxidative stress. Free Radic. Biol. Med. 1998, 24, 66–75. [Google Scholar] [CrossRef]
- Kamal, A.; Hussaini, S.M.; Malik, M.S. Recent developments towards podophyllotoxin congeners as potential apoptosis inducers. Anticancer Agents Med. Chem. 2015, 15, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Sen, V.D.; Terentiev, A.A.; Konovalova, N.P. Platinum complexes with bioactive nitroxyl radicals: Synthesis and antitumor properties. In Nitroxides-Theory, Experiment and Applications; Kokorin, A., Ed.; InTech: London, UK, 2012; pp. 385–406. ISBN 978-953-51-0722-4. [Google Scholar]
- Yoshitomi, T.; Ozaki, Y.; Thangavel, S.; Nagasaki, Y. Redox nanoparticle therapeutics to cancer—Increase in therapeutic effect of doxorubicin, suppressing its adverse effect. J. Controll. Release 2013, 172, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, O.D.; Frolova, T.S.; Yushkova, Y.V.; Chernyak, E.I.; Pokrovsky, A.G.; Pokrovsky, M.A.; Morozov, S.V.; Sinitsina, O.I.; Grigor’ev, I.A.; Nevinsky, G.A. Antioxidant and antitumor activity of trolox, trolox succinate, and α-tocopheryl succinate conjugates with nitroxides. Eur. J. Med. Chem. 2016, 122, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Stanley, B.A.; Sivakumaran, V.; Kembro, J.M.; O’Rourke, B.; Paolocci, N.; Cortassa, S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: An experimental-computational study. J. Gen. Physiol. 2012, 139, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. The respiratory burst of phagocytes. J. Clin. Investig. 1984, 73, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Carreras, M.C.; Riobó, N.A.; Pargament, G.A.; Boveris, A.; Poderoso, J.J. Effects of respiratory burst inhibitors on nitric oxide production by human neutrophils. Free Radic. Res. 1997, 26, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Mach, W.J.; Thimmesch, A.R.; Pierce, J.T.; Pierce, J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs. Res. Pract. 2011, 2011, 260482. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Ramirez-Boscá, A.; Soler, A.; Díez, A.; Carrión-Gutiérrez, M.A.; Díaz-Alperi, J.; Quintanilla-Ripoll, E.; Bernd, A.; Quintanilla-Almagro, E. Increase with age of serum lipid peroxides: Implications for the prevention of atherosclerosis. Mech. Ageing Dev. 1998, 100, 17–24. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; Ruiz-Ramos, M.; Sánchez-Rodríguez, M.A.; Retana-Ugalde, R.; Muñoz-Sánchez, J.L. Aging-related oxidative stress in healthy humans. Tohoku J. Exp. Med. 2007, 213, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Siomek, A.; Gackowski, D.; Rozalski, R.; Dziaman, T.; Szpila, A.; Guz, J.; Olinski, R. Higher leukocyte 8-oxo-7,8-dihydro-2′-deoxyguanosine and lower plasma ascorbate in aging humans? Antioxid. Redox Signal. 2007, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.A.; Woulfe, J. Lipofuscin and aging: A matter of toxic waste. Sci. Aging Knowl. Environ. 2005, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Schroder, P.; Biesalski, H.K. Low Molecular Weight Antioxidants. Handb. Environ. Chem. 2005, 2, 77–90. [Google Scholar]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
- Groeger, G.; Quiney, C.; Cotter, T.G. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Signal. 2009, 11, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014, 2, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J. Caloric restriction and aging: An update. Exp. Gerontol. 2000, 35, 299–305. [Google Scholar] [CrossRef]
- Durstine, J.L.; Gordon, B.; Wang, Z.; Luo, X. Chronic disease and the link to physical activity. J. Sport Health Sci. 2013, 2, 3–11. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Photoprotective and radioprotective properties of nitroxides and their application in magnetic resonance imaging. Postepy Hig. Med. Doswiadczalnej 2016, 70, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; Abouzied, M.M.; Mohafez, O.M. Tempol ameliorates cardiac fibrosis in streptozotocin-induced diabetic rats: Role of oxidative stress in diabetic cardiomyopathy. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.C.V.; Cerdeira, C.D.; Camara, E.P.; Garcia, J.A.D.; Brigagão, M.R.P.L.; Silva, R.B.V.; Dos Santos, G.B. Tempol improves lipid profile and prevents left ventricular hypertrophy in LDL receptor gene knockout (LDLr-/-) mice on a high-fat diet. Rev. Port. Cardiol. 2017, 36, 629–638. [Google Scholar]
- Thiemermann, C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit. Care Med. 2003, 31, S76–S84. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Yanaka, K.; Hyodo, K.; Homma, K.; Nagase, S.; Nose, T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003, 979, 188–193. [Google Scholar] [CrossRef]
- Aksu, U.; Ergin, B.; Bezemer, R.; Kandil, A.; Milstein, D.M.; Demirci-Tansel, C.; Ince, C. Scavenging reactive oxygen species using tempol in the acute phase of renal ischemia/reperfusion and its effects on kidney oxygenation and nitric oxide levels. Intensive Care Med. Exp. 2015, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Pınar, N.; Soylu, K.O.; Özcan, O.; Atik Doğan, E.; Bayraktar, S. Protective effects of tempol in an experimental ovarian ischemia-reperfusion injury model in female Wistar albino rats. Can. J. Physiol Pharmacol. 2017, 95, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, U.; Christensen, F.H.; Buus, N.H. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol. Ther. 2009, 122, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, U.; Rodriguez-Rodriguez, R.; Dalsgaard, T.; Buus, N.H.; Stankevicius, E. Novel approaches to improving endothelium-dependent nitric oxide-mediated vasodilatation. Pharmacol. Rep. 2009, 61, 105–115. [Google Scholar] [CrossRef]
- Weindruch, R. Caloric restriction and aging. Sci. Am. 1996, 274, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Xavier, S.; DeLuca, A.M.; Sowers, A.L.; Cook, J.A.; Krishna, M.C.; Hahn, S.M.; Russo, A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic. Biol. Med. 2003, 34, 93–102. [Google Scholar] [CrossRef]
- Samuni, Y.; Cook, J.A.; Choudhuri, R.; Degraff, W.; Sowers, A.L.; Krishna, M.C.; Mitchell, J.B. Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic. Biol. Med. 2010, 49, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.J.; Mitchell, J.B.; Bursill, C.A.; Sowers, A.L.; Thetford, A.; Cook, J.; van Reyk, D.M.; Davies, M.J. The nitroxide radical TEMPOL prevents obesity, hyperlipidaemia, elevation of inflammatory cytokines, and modulates atherosclerotic plaque composition in apoE-/-mice. Atherosclerosis 2015, 240, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Ishimatsu, A.; Yuuki Yamanaka, Y.; Mine, T.; Yamada, K. Tempol intake improves inflammatory status in aged mice. J. Clin. Biochem. Nutr. 2014, 55, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Park, C.W.; Shin, S.J.; Lim, J.H.; Chung, H.W.; Youn, D.Y.; Kim, H.W.; Kim, B.S.; Lee, J.H.; Kim, G.H.; et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol. Dial. Transplant. 2010, 25, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.F.; Yuan, J.; Roy, S.; Imig, J.D. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am. J. Physiol. Ren. Physiol. 2010, 298, F86–F94. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Yoshizumi, M.; Hitomi, H.; Kagami, S.; Kondo, S.; Miyatake, A.; Fukunaga, M.; Tamaki, T.; Kiyomoto, H.; Kohno, M.; et al. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J. Am. Soc. Nephrol. 2004, 15, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Yao, L.; Nagai, Y.; Miyata, K.; Yoshizumi, M.; Kagami, S.; Kondo, S.; Kiyomoto, H.; Shokoji, T.; Kimura, S.; et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 2004, 43, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Filipe, H.M.; Costantino, G.; Mazzon, E.; Santagati, S.; Caputi, A.P.; Thiemermann, C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur. J. Pharmacol. 2000, 390, 209–222. [Google Scholar] [CrossRef]
- Zarling, J.A.; Brunt, V.E.; Vallerga, A.K.; Li, W.; Tao, A.; Zarling, D.A.; Minson, C.T. Nitroxide pharmaceutical development for age-related degeneration and disease. Front. Genet. 2015, 6, 325. [Google Scholar] [CrossRef] [PubMed]
- Buschini, E.; Fea, A.M.; Lavia, C.A.; Nassisi, M.; Pignata, G.; Zola, M.; Grignolo, F.M. Recent developments in the management of dry age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.Q.; Godley, B.F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef]
- Zhou, J.; Jang, Y.P.; Chang, S.; Sparrow, J.R. OT-674 suppresses photooxidative processes initiated by an RPE lipofuscin fluorophore. Photochem. Photobiol. 2008, 84, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Reinke, M.C.; Brunt, V.E.; Minson, C.T. Impaired acetylcholine-induced cutaneous vasodilation in young smokers: Roles of nitric oxide and prostanoids. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H667–H673. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Brunt, V.E.; Minson, C.T. Tempol improves cutaneous thermal hyperemia through increasing nitric oxide bioavailability in young smokers. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1507–H1511. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Hwang, S.H.; Abdi, S. Tempol ameliorates and prevents mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain. Front. Pharmacol. 2017, 7, 532. [Google Scholar] [CrossRef] [PubMed]











© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowski, M.; Gwozdzinski, K. Nitroxides as Antioxidants and Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 2490. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18112490
Lewandowski M, Gwozdzinski K. Nitroxides as Antioxidants and Anticancer Drugs. International Journal of Molecular Sciences. 2017; 18(11):2490. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18112490
Chicago/Turabian StyleLewandowski, Marcin, and Krzysztof Gwozdzinski. 2017. "Nitroxides as Antioxidants and Anticancer Drugs" International Journal of Molecular Sciences 18, no. 11: 2490. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18112490