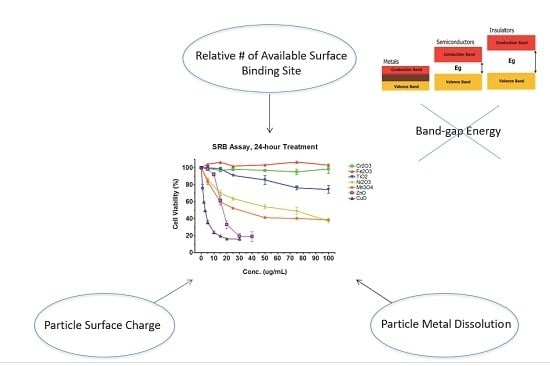
The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms
Abstract
:1. Introduction
2. Characteristics of Nanoparticles that Influence Toxicity
3. Biochemical and Molecular Mechanisms of Cytotoxicity
4. Mechanisms of Cell Cycle Arrest
4.1. Cell-Type-Dependent Suppression of the Cell Cycle
4.2. Nanoparticle Dependent Suppression of Cell Cycle
4.3. Changes in Gene Expression Underlie the Mechanisms of Cell Cycle Arrest
5. Cytotoxicity Is a Function of Cell Killing and Suppression of Proliferation
6. Conclusions
Conflicts of Interest
References
- Jones, B.J.; Vergne, M.J.; Bunk, D.M.; Locascio, L.E.; Hayes, M.A. Cleavage of Peptides and Proteins Using Light-Generated Radicals from Titanium Dioxide. Anal. Chem. 2007, 79, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- TIME. TIME’s Best Inventions of 2008. Available online: http://content.time.com/time/specials/packages/article/0,28804,1852747_1854195_1854176,00.html (accessed on 19 October 2017).
- Earle, M.D. The Electrical Conductivity of Titanium Dioxide. Phys. Rev. 1942, 61, 56–62. [Google Scholar] [CrossRef]
- Hogan, J. Smog-busting paint soaks up noxious gases. New Scientist, 4 February 2004. [Google Scholar]
- Phillips, L.G.; Barbano, D.M. The Influence of Fat Substitutes Based on Protein and Titanium Dioxide on the Sensory Properties of Lowfat Milks. J. Dairy Sci. 1997, 80, 2726–2731. [Google Scholar] [CrossRef]
- Fujishima, A. Discovery and applications of photocatalysis—Creating a comfortable future by making use of light energy. Jpn. Nanonet Bull. 2005, 44, 1–3. [Google Scholar]
- Fujishmia, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Balaji, K.; Rao, K.J. Microwave-Assisted Solid-State Synthesis of Oxide Ion Conducting Stabilized Bismuth Vanadate Phases. Chem. Mater. 1998, 10, 3400–3404. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK; Boston, MA, USA, 1997. [Google Scholar]
- Page, E.M.; Wass, S.A. Vanadium:Inorganic and Coordination chemistry. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Adlam, G.H.J.; Price, L.S. Higher School Certificate Inorganic Chemistry; Anybook Ltd.: Lincoln, UK, 1945. [Google Scholar]
- Greedon, J.E. Magnetic oxides. In Encyclopedia of Inorganic Chemistry; King, R.B., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Babes, L.; Denizot, B.; Tanguy, G.; Jacques Le Jeunne, J.; Jallet, P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J. Colloid Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Encyclopedia of Chemical Processing; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Buxbaum, G.; Pfaff, G. Industrial Inorganic Pigments, 3rd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- AZoNano. Copper Oxide (CuO) Nanoparticles—Properties, Applications. Available online: https://www.azonano.com/article.aspx?ArticleID=3395 (accessed on 21 October 2017).
- Kenney, C.W.; Uchida, L.A. Use of Copper (II) Oxide as Source of Oxygen for Oxidation Reactions. Available online: http://www.freepatentsonline.com/4582613.html (accessed on 21 October 2017).
- Saito, M. Antibacterial, Deodorizing, and UV Absorbing Materials Obtained with Zinc Oxide (ZnO) Coated Fabrics. J. Ind. Text. 1993, 23, 150–164. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.-L.; Jiang, W.-C. Durability of nano ZnO antibacterial cotton fabric to sweat. J. Appl. Polym. Sci. 2007, 103, 412–416. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Enhancement of antibacterial properties of Ag nanorods by electric field. Sci. Technol. Adv. Mater. 2009, 10, 015003. [Google Scholar] [CrossRef] [PubMed]
- AZoNano. Zinc Oxide (ZnO) Nanoparticles—Properties, Applications. Available online: https://www.azonano.com/article.aspx?ArticleID=3348 (accessed on 21 October 2017).
- Li, Y.B.; Bando, Y.; Golberg, D. ZnO nanoneedles with tip surface perturbations: Excellent field emitters. Appl. Phys. Lett. 2004, 84, 3603–3605. [Google Scholar] [CrossRef]
- Kumar, S.A.; Chen, S.M. Nanostructured Zinc Oxide Particles in Chemically Modified Electrodes for Biosensor Applications. Anal. Lett. 2008, 41, 141–158. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Lin Wang, Z. Editor’s summary: Nanomaterial: Power dresser. Nature 2008, 451, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Korin, N.; Kanapathipillai, M.; Ingber, D.E. Sheer-responsive platemet mimetics for targeted drug delivery. Isr. J. Chem. 2012, 53, 610–615. [Google Scholar]
- Highsmith, J. Nanoparticles in Biotechnology, Drug Development and Drug Delivery. In Global Markets: A BCC Research Report; BCC Research: Wellesley, MA, USA, 2014. [Google Scholar]
- Leso, V.; Fontana, L.; Mauriello, M.C.; Iavicoli, I. Occupational risk assessment of engineered nanomaterials challenges and opportunities. Curr. Nanosci. 2017, 13, 55–78. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdorster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does Nanoparticle Activity Depend upon Size and Crystal Phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Xiaojun, X.; Ximing, P.; Zhenqing, H.; Qiqing, Z. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 2011, 6, 480. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors influencing the cytotoxicity of zinc oxide nanoparticles: Particle size and surface charge. J. Phys. Conf. Ser. 2011, 304, 012044. [Google Scholar] [CrossRef]
- Huang, Y.W.; Lee, H.J.; Tolliver, L.M.; Aronstam, R.S. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: Opportunities and challenges. BioMed Res. Int. 2015, 2015, 834079. [Google Scholar] [CrossRef] [PubMed]
- Chusuei, C.C.; Wu, C.H.; Mallavarapu, S.; Hou, F.Y.; Hsu, C.M.; Winiarz, J.G.; Aronstam, R.S.; Huang, Y.W. Cytotoxicity in the age of nano: The role of fourth period transition metal oxide nanoparticle physicochemical properties. Chem.-Biol. Interact. 2013, 206, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, J.; Xue, Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol. Lett. 2010, 199, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Leclerc, L.; Hochepie, J.F.; Trouvé, A.; Sarry, G.; Pourchez, J. Impact of Cerium Oxide Nanoparticles Shape on their In Vitro Cellular Toxicity. Toxicol. In Vitro 2017, 38, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Delorme, M.P.; Muro, Y.; Arai, T.; Banas, D.A.; Frame, S.R.; Reed, K.L.; Warheit, D.B. Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats. Toxicol. Sci. 2012, 128, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Guttenberg, M.; Bezerra, L.; Neu-Baker, N.M.; del Pilar Sosa Idelchik, M.; Elder, A.; Oberdorster, G.; Brenner, S.A. Biodistribution of inhaled metal oxide nanoparticles mimicking occupational exposure: A preliminary investigation using enhanced darkfield microscopy. J. Biophotonics 2016, 9, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Webb, T.R.; Colvin, V.L.; Reed, K.L.; Sayes, C.M. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicol. Sci. 2007, 95, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Miller, M.R.; Clift, M.J.D.; Elder, A.; Mills, N.L.; Møller, P.; Schins, R.P.F.; Vogel, U.; Kreyling, W.G.; Alstrup Jensen, K.; et al. Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, Y.; Huang, C.-C.; Ma, Y.; Shannon, K.B.; Chen, D.-R.; Huang, Y.-W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009, 11, 25–39. [Google Scholar] [CrossRef]
- Huang, C.C.; Aronstam, R.S.; Chen, D.R.; Huang, Y.W. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. In Vitro 2010, 24, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Stayton, I.; Huang, Y.-W.; Zhou, X.-D. Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549. Toxicol. Environ. Chem. 2008, 90, 983–996. [Google Scholar] [CrossRef]
- Wang, H.J.; Growcock, A.C.; Tang, T.H.; O’Hara, J.; Huang, Y.W.; Aronstam, R.S. Zinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signaling pathway. Toxicol. In Vitro 2010, 24, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.H.; Chang, C.T.; Wang, H.J.; Erickson, J.D.; Reichard, R.A.; Martin, A.G.; Shannon, E.K.; Martin, A.L.; Huang, Y.W.; Aronstam, R.S. Oxidative stress disruption of receptor-mediated calcium signaling mechanisms. J. Biomed. Sci. 2013, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wei, Y.; Zhao, H.; Chen, S.; Bu, X.; Lu, F.; Qu, D.; Yao, L.; Zheng, J.; Zhang, J. The effect of Fe2O3 and ZnO nanoparticles on cytotoxicity and glucose metabolism in lung epithelial cells. J. Appl. Toxicol. 2015, 35, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. Int. 2015, 22, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.S.; Athinarayanan, J.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Fe3O4 nanoparticle redox system modulation via cell-cycle progression and gene expression in human mesenchymal stem cells. Environ. Toxicol. 2016, 31, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ma, N.; Zhou, H.; Wang, Q.; Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Li, L. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 11, 3859–3874. [Google Scholar]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Capasso, L.; Camatini, M.; Gualtieri, M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol. Lett. 2014, 226, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C.; Chen, W.; Li, X. Comparative toxicities of bismuth oxybromide and titanium dioxide exposure on human skin keratinocyte cells. Chemosphere 2015, 135, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N.; Zhuang, F.; Connolly, S.; Li, J.; Ogawa, N.; Xu, M. Molecular Responses of Human Lung EpithelialCellstotheToxicityofCopper Oxide Nanoparticles Inferred from Whole Genome Expression Analysis. ACS Nano 2011, 5, 9326–9338. [Google Scholar] [CrossRef] [PubMed]
- Moschini, E.; Gualtieri, G.; Gallinotti, D.; Pezzolato, E.; Fascio, U.; Camatini, M.; Mantecca, P. Metal oxide nanoparticles induce cytotoxic effects on human lung epithelial cells A549. Chem. Eng. Trans. 2010, 22, 29–34. [Google Scholar]
- Kansara, K.; Patel, P.; Shah, D.; Shukla, R.K.; Singh, S.; Kumar, A.; Dhawan, A. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ. Mol. Mutagen. 2015, 56, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.; Yang, L.; Zheng, Y.; Long, J.; Jia, J.; Xiao, S.; Liu, J. Activation of Erk and p53 regulates copper oxide nanoparticle-induced cytotoxicity in keratinocytes and fibroblasts. Int. J. Nanomed. 2014, 9, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Aluminum oxide nanoparticles alter cell cycle progression through CCND1 and EGR1 gene expression in human mesenchymal stem cells. Biotechnol. Appl. Biochem. 2016, 63, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Kansara, K.; Senapati, V.A.; Shanker, R.; Dhawan, A.; Kumar, A. Cell cycle dependent cellular uptake of zinc oxide nanoparticles in human epidermal cells. Mutagenesis 2016, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Costa, C.; Kilic, G.; Costa, S.; Pasaro, E.; Laffon, B.; Teixeira, J.P. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ. Int. 2013, 55, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fujita, D.; Kajiwara, S.; Minowa, T.; Li, X.; Takemura, T.; Iwai, H.; Hanagata, N. Contribution of physicochemical characteristics of nano-oxides to cytotoxicity. Biomaterials 2010, 31, 8022–8031. [Google Scholar] [CrossRef] [PubMed]
- Thit, A.; Selck, H.; Bjerregaard, H.F. Toxicity of CuO nanoparticles and Cu ions to tight epithelial cells from Xenopus laevis (A6): Effects on proliferation, cell cycle progression and cell death. Toxicol. In Vitro 2013, 27, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Mechanistic Investigation of the Biological Effects of SiO2, TiO2, and ZnO Nanoparticles on Intestinal Cells. Small 2015, 11, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Tolliver, L.; Cambre, M.; Hou, F.Y.; Lee, H.J.; Aronstam, R.; Huang, Y.W. Nanotoxicity of Transition Metal Oxides is a Function of Cell Killing and Suppression of Cell Proliferation. Toxicol. In Vitro 2017. In preparation. [Google Scholar]


| Elements | Oxide | Potential Application |
|---|---|---|
| Scandium (Sc) | Sc2O3 | Used in high-temperature systems for its resistance to heat and thermal shock, electronic ceramics, and glass composition |
| Titanium (Ti) [1,2,3,4,5,6,7] | TiO2 | White pigment, white food coloring, cosmetic and skin care products, thickener, tattoo pigment and styptic pencils, plastics, semiconductor, solar energy conversion, solar cells, solid electrolytes, detoxification or remediation of wastewater; used in resistance-type lambda probes; can be used to cleave protein that contains the amino acid proline at the site where proline is present, and as a material in the meristor |
| Vanadium (V) | V2O5 | Catalyst, a detector material in bolometers and microbolometer arrays for thermal imaging, and in the manufacture of sulfuric acid, vanadium redox batteries; preparation of bismuth vanadate ceramics for use in solid oxide fuel cells [8] |
| V2O3 | Corundum structure as an abrasive [9], antiferromagnetic with a critical temperature at 160 K [10] can change in conductivity from metallic to insulating | |
| Chromium (Cr) | Cr2O3 | Protection of silicon surface morphology during deep ion coupled plasma etching of silica layers; used in paints, inks, and is the precursor to the magnetic pigment chromium dioxide |
| CrO2 | Magnetic tape emulsion, data tape applications | |
| Manganese (Mn) | MnO2 | Electrochemical capacitor, as a catalyst; used in industrial water treatment plants |
| Iron (Fe) | Fe2O3 | Used as contrast agents in magnetic resonance imaging, in labeling of cancerous tissues, magnetically controlled transport of pharmaceuticals, localized thermotherapy, preparation of ferrofluids [11,12], final polish on metallic jewelry and lenses, as a cosmetic |
| FeO | Tattoo inks | |
| Fe3O4 | MRI scanning [13], as a catalyst in the Haber process and in the water gas shift reaction [14], and as a black pigment [15] | |
| Cobalt (Co) | Co2O3 | Catalyst; for studying the redox and electron transfer properties of biomolecules; can immobilize protein |
| CoO | Blue colored glazes and enamels, producing cobalt(II) salts | |
| Nickel (Ni) | NiO | In ceramic structures, materials for temperature or gas sensors, nanowires and nanofibers, active optical filters, counter electrodes |
| Ni2O3 | Electrolyte in nickel plating solutions; an oxygen donor in auto emission catalysts; forms nickel molybdate, anodizing aluminum, conductive nickel zinc ferrites; in glass frit for porcelain enamel; thermistors, varistors, cermets, and resistance heating element | |
| Copper (Cu) | CuO | Burning rate catalyst, superconducting materials, thermoelectric materials, catalysts, sensing materials, glass, ceramics, ceramic resisters, magnetic storage media, gas sensors, near infrared tilters, photoconductive applications, photothermal applications, semiconductors, solar energy transformation [16]; can be used to safely dispose of hazardous materials [17] |
| Cu2O | Pigment, fungicide, antifouling agent for marine paints, semiconductor | |
| Zinc (Zn) | ZnO | Added to cotton fabric, rubber, food packaging [18,19,20], cigarettes [21], field emitters [22], nanorod sensors; Applications in laser diodes and light emitting diodes (LEDs), a biomimic membrane to immobilize and modify biomolecules [23]; increased mechanical stress of textile fibers [24] |
| Cell Line | Nanoparticle | Size (nm) | Specific Surface Area (m2/g) | Zeta Potential (mV) | Shape | Effect on Cell Cycle | Reference |
|---|---|---|---|---|---|---|---|
| Human alveolar adenocarcinoma (A549) | TiO2 | >100 | --- | --- | irregular | ↑G0/G1 | [59] |
| A549 | Fe2O3 | 39.2 * | --- | --- | spherical | No change | [50] |
| A549 | CuO | 50 | --- | −23.96 ** | sphere | ↑G2/M | [58,66] |
| A549 | CuO | >50 | --- | --- | irregular | ↑G2/M | [59] |
| A549 | NiO | 50, 80 *, 450 ** | 61.16 | −12; −22 | --- | ↑G0/G1 | [56] |
| ↑G2/M | |||||||
| ↑sub G0 | |||||||
| A549 | ZnO | 63.1 * | --- | --- | nearly spherical | ↑G2/M | [50] |
| A459 | TiO2 | 23.28 ± 2.0 ** | 12–15 | −10.16 ± 1.0 ** | anatase | ↑G2/M | [60] |
| 106.7 ± 8.0 * | |||||||
| 4–8 | −13 ± 0.9 * | ||||||
| A549 | TiO2 | <5 | 200 | −0.55 ** | anatase | ↑G2/M | [51] |
| 65.3 ** | ↓G0/G1 | ||||||
| Human bronchial epithelial cells (BEAS-2B) | Fe2O3 | 39.2 * | --- | --- | spherical | No change | [50] |
| BEAS-2B | NiO | 50 | --- | −12/−22 | --- | ↑G0/G1 | [56] |
| ↓G2/M | |||||||
| ↓S | |||||||
| ↑Sub G0 | |||||||
| BEAS-2B | ZnO | 63.1 * | --- | --- | nearly spherical | No change | [50] |
| Human immortal keratinocyte cells (HaCaT) | TiO2 | 12 ** | --- | −11.9 ± 0.8 ** | spherical | ↓G0/G1 | [57] |
| ↑S | |||||||
| HaCaT | ZnO | <100 | 15–25 | −12.6 ± 0.95 ** | rod-shaped | ↑G2/M | [53] |
| 132.55 ± 0.45 ** | ↓S | ||||||
| HaCaT | CuO | 3–6 * | --- | ~37.5 * | --- | ↑G2/M | [61] |
| ↓G0/G1 | |||||||
| ↓S | |||||||
| Rat pheochromocytoma (PC12) | TiO2 | 20 | --- | −12.5 | anatase | ↑G2/M | [33] |
| Rat PC12 | TiO2 | 20 | --- | −23.2 | rutile | ↑G2/M | [33] |
| Human neuroplastoma (SHSY5Y) | ZnO | 100 | 15–20 | −8.23 * | --- | ↓G0/G1 | [65] |
| 243.7 * | −11.7 ** | ↓G2/M | |||||
| 273.4 ** | ↑S | ||||||
| Human mesenchymal stem cells (hMSFs) | Al2O3 | 20–100 | --- | --- | spherical | ↓G0/G1 | [62] |
| ↓G2/M | |||||||
| 205 * | ↑Sub G0 | ||||||
| Human hMSFs | Fe3O4 | 50–75 | --- | --- | spherical | ↓G0/G1 | [52] |
| 119 * | ↑Sub G0 | ||||||
| 210 ** | |||||||
| Human hepatoma (BEL-7402) | Fe3O4 | 10–30 | --- | 14.4 | --- | ↑G0/G1 | [29] |
| ↓S | |||||||
| Human epidermal carcinoma (A431) | ZnO | 215.8 ± 0.1 * | --- | −25.3 ± 0.4 * | --- | ↑S | [64] |
| 30.9 ± 0.5 * | −12.8 ± 0.6 ** | ↑G2/M | |||||
| Allium cepa root cells | ZnO | 75–85 | --- | --- | mostly cuboidal to hexagonal-octagonal, some rod | ↓G0/G1 | [63] |
| ↑G2/M | |||||||
| ↑Sub G0 | |||||||
| Mouse embryonic fibroblast (MEF) | CuO | 3–6 | --- | ~37.5 | --- | ↑G2/M | [61] |
| ↓G0/G1 | |||||||
| ↓S | |||||||
| Xenopus laevis (A6) | Poly-CuO | 100 | --- | --- | --- | ↑G2/M | [67] |
| 40–500 * | ↓S | ||||||
| Xenopus laevis (A6) | CuO | 6 ± 1 | --- | --- | --- | ↑G2/M | [67] |
| 9–40 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-W.; Cambre, M.; Lee, H.-J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122702
Huang Y-W, Cambre M, Lee H-J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. International Journal of Molecular Sciences. 2017; 18(12):2702. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122702
Chicago/Turabian StyleHuang, Yue-Wern, Melissa Cambre, and Han-Jung Lee. 2017. "The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms" International Journal of Molecular Sciences 18, no. 12: 2702. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122702