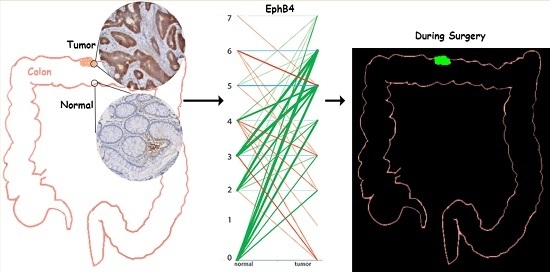
Evaluation of EphA2 and EphB4 as Targets for Image-Guided Colorectal Cancer Surgery
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Tissue Micro Array (TMA)
4.2. Immunohistochemistry
4.3. Scoring Method
4.4. Cell Culture, Flow Cytometry, and Chamber Slide Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IGOS | Image-guided oncologic surgery |
| NIH | National Institutes of Health |
| TMA | Tissue microarray |
| IHC | Immunohistochemistry |
| FFPE | Formalin-fixed paraffin-embedded |
| PET | Positron emission tomography |
References
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Kerrebijn, J.D.; van Driel, P.B.; Xie, B.; Kaijzel, E.L.; Snoeks, T.J.; Que, I.; Hutteman, M.; van der Vorst, J.R.; Mieog, J.S.; et al. Optical image-guided surgery—Where do we stand? Mol. Imag. Biol. 2011, 13, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; de Geus, S.W.; Prevoo, H.A.; Hawinkels, L.J.; van de Velde, C.J.; Kuppen, P.J.; Vahrmeijer, A.L.; Sier, C.F. Selecting targets for tumor imaging: An overview of cancer-associated membrane proteins. Biomark. Cancer 2016, 8, 15. [Google Scholar]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Herath, N.I.; Boyd, A.W. The role of Eph receptors and Ephrin ligands in colorectal cancer. Int. J. Cancer J. Int. Du Cancer 2010, 126, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.Q.; Wu, X.S.; Wei, B.; Chen, L. Eph receptors and Ephrins as targets for cancer therapy. J. Cell. Mol. Med. 2012, 16, 2894–2909. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptors and Ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.D.; Dasgupta, S.; Blayney, J.K.; McArt, D.G.; Redmond, K.L.; Weir, J.A.; Bradley, C.A.; Sasazuki, T.; Shirasawa, S.; Wang, T.; et al. EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin. Cancer Res. 2016, 22, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Igarashi, H.; Kanamori, M.; Ihara, M.; Wang, J.D.; Wang, Y.J.; Li, Z.Y.; Shimamura, T.; Kobayashi, T.; Maruyama, K.; et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004, 95, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Reggiani Bonetti, L.; Ieni, A.; Branca, G.; Tuccari, G. Histologic prognostic markers in Stage IIA colorectal cancer: A comparative study. Scand. J. Gastroenterol. 2016, 51, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.; Fenoglio-Preiser, C.M.; Pettigrew, N.; Fielding, L.P. American joint committee on cancer prognostic factors consensus conference: Colorectal working group. Cancer 2000, 88, 1739–1757. [Google Scholar] [CrossRef]
- Herath, N.I.; Spanevello, M.D.; Doecke, J.D.; Smith, F.M.; Pouponnot, C.; Boyd, A.W. Complex expression patterns of Eph receptor tyrosine kinases and their Ephrin ligands in colorectal carcinogenesis. Eur. J. Cancer 2012, 48, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Barquilla, A.; Pasquale, E.B. Eph receptors and Ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.A.; Slomka, S.; Douglas, E.L.; Hewett, P.J.; Hardingham, J.E. Receptor protein tyrosine kinase EphB4 is upregulated in colon cancer. BMC Mol. Biol. 2001, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Batlle, E.; Bacani, J.; Begthel, H.; Jonkheer, S.; Gregorieff, A.; van de Born, M.; Núria, M.; Elena, S.; Elles, B.; Tony, P.; et al. EphB receptor activity suppresses colorectal cancer progression. Nature 2005, 435, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Vemula, S.V.; Mittal, S.K. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets 2011, 15, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Masood, R.; Spannuth, W.A.; Singh, J.; Scehnet, J.; Kleiber, G.; Jennings, N.; Deavers, M.; Krasnoperov, V.; Dubeau, L.; et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br. J. Cancer 2007, 96, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Fujimoto, J.; Jahan, I.; Sato, E.; Tamaya, T. Coexpression of EphB4 and EphrinB2 in tumour advancement of ovarian cancers. Br. J. Cancer 2008, 98, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Munoz, I.; Sanchez, A.; Martinez-Martinez, E.; Garcia, J.M.; Salas, C.; Provencio, M.; Alvarez-Vallina, L.; Sanz, L. Gene expression profiling identifies EPHB4 as a potential predictive biomarker in colorectal cancer patients treated with bevacizumab. Med. Oncol. 2013, 30, 572. [Google Scholar] [CrossRef] [PubMed]
- Davalos, V.; Dopeso, H.; Castano, J.; Wilson, A.J.; Vilardell, F.; Romero-Gimenez, J.; Eloy, E.; Manel, A.; Gabriel, C.; John, M.M. EPHB4 and survival of colorectal cancer patients. Cancer Res. 2006, 66, 8943–8948. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Miao, H.; Gerber, L.; Singh, J.; Denning, M.F.; Gilliam, A.C.; Wang, B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006, 66, 7050–7058. [Google Scholar] [CrossRef] [PubMed]
- Yeddula, N.; Xia, Y.; Ke, E.; Beumer, J.; Verma, I.M. Screening for tumor suppressors: Loss of Ephrin receptor A2 cooperates with oncogenic KRas in promoting lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, E6476–E6485. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Park, R.; Liu, R.; Xia, Z.; Guo, J.; Krasnoperov, V.; Gill, P.S.; Li, Z.; Shan, H.; et al. PET imaging of colorectal and breast cancer by targeting EphB4 receptor with 64Cu-labeled hAb47 and hAb131 antibodies. J. Nucl. Med. 2013, 54, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Pasquale, E.B. Targeting the Eph system with peptides and peptide conjugates. Curr. Drug Targets 2015, 16, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, N.; Fleming, R.; Hay, C.; Woods, R.; Xu, L.; Wu, H.; Gao, C. Development of a trispecific antibody designed to simultaneously and efficiently target three different antigens on tumor cells. Mol. Pharm. 2015, 12, 3490–3501. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, S.; Liu, R.; Park, R.; Hughes, L.; Krasnoperov, V.; Gill, P.S.; Li, Z.; Shan, H.; Conti, P.S. Targeting the EphB4 receptor for cancer diagnosis and therapy monitoring. Mol. Pharm. 2013, 10, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kijima, S.; Sasaki, T.; Nagata, K.; Utano, K.; Lefor, A.T.; Sugimoto, H. Preoperative evaluation of colorectal cancer using CT colonography, MRI, and PET/CT. World J. Gastroenterol. 2014, 20, 16964–16975. [Google Scholar] [CrossRef] [PubMed]
- Torok, J.A.; Palta, M.; Willett, C.G.; Czito, B.G. Nonoperative management of rectal cancer. Cancer 2016, 122, 34–41. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Group | Percentage (%) |
|---|---|---|
| Gender | Male | 48.1 |
| Female | 51.9 | |
| Age | <50 | 11.3 |
| ≥50 | 88.7 | |
| Stage | I | 20.3 |
| IIA | 25.0 | |
| IIB | 1.9 | |
| IIIA | 3.8 | |
| IIIB | 21.8 | |
| IIIC | 8.3 | |
| IV | 18.6 | |
| Differentiation | Good | 25.7 |
| Moderate | 64.2 | |
| Poor | 10.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stammes, M.A.; Prevoo, H.A.J.M.; Ter Horst, M.C.; Groot, S.A.; Van de Velde, C.J.H.; Chan, A.B.; De Geus-Oei, L.-F.; Kuppen, P.J.K.; Vahrmeijer, A.L.; Pasquale, E.B.; et al. Evaluation of EphA2 and EphB4 as Targets for Image-Guided Colorectal Cancer Surgery. Int. J. Mol. Sci. 2017, 18, 307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18020307
Stammes MA, Prevoo HAJM, Ter Horst MC, Groot SA, Van de Velde CJH, Chan AB, De Geus-Oei L-F, Kuppen PJK, Vahrmeijer AL, Pasquale EB, et al. Evaluation of EphA2 and EphB4 as Targets for Image-Guided Colorectal Cancer Surgery. International Journal of Molecular Sciences. 2017; 18(2):307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18020307
Chicago/Turabian StyleStammes, Marieke A., Hendrica A. J. M. Prevoo, Meyke C. Ter Horst, Stéphanie A. Groot, Cornelis J. H. Van de Velde, Alan B. Chan, Lioe-Fee De Geus-Oei, Peter J. K. Kuppen, Alexander L. Vahrmeijer, Elena B. Pasquale, and et al. 2017. "Evaluation of EphA2 and EphB4 as Targets for Image-Guided Colorectal Cancer Surgery" International Journal of Molecular Sciences 18, no. 2: 307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18020307