Effects of Remote Ischemic Preconditioning on Heme Oxygenase-1 Expression and Cutaneous Wound Repair
Abstract
:1. Introduction
2. Results
2.1. Effects of RIPC on HO-1 Promoter Activity and HO-1 mRNA Expression in Mice
2.2. Effects of Early or Late RIPC on Excisional Cutaneous Wound Closure in Mice
2.3. HO-1 mRNA and Protein Expression in Wounds After RIPC
2.4. Effects of RIPC on Wound Morphology and Collagen Deposition
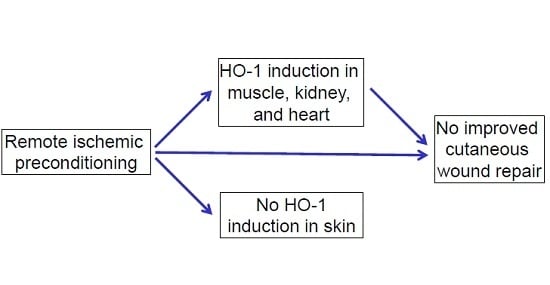
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RIPC Treatment
4.3. Measuring of HO-1 Promoter Activity
4.4. Excisional Wound Model
4.5. Sample Collection
4.6. (Immuno-)histochemical Staining and Analyses
4.7. RNA Isolation and Quantitative-RT-PCR
4.8. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| H&E | hematoxylin and eosin |
| HO-1 | heme oxygenase-1 |
| HO-1-luc Tg | HO-1-luciferase transgenic |
| I/R | Ischemia/Reperfusion |
| IPC | Ischemic preconditioning |
| IRI | Ischemia/Reperfusion injury |
| RIPC | Remote ischemic preconditioning |
| RPCT | Remote preconditioning by trauma |
| ROS | Reactive oxygen species |
Appendix A



References
- Rabello, F.B.; Souza, C.D.; Farina Junior, J.A. Update on hypertrophic scar treatment. Clinics 2014, 69, 565–573. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef] [PubMed]
- Sidgwick, G.P.; Bayat, A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. Interaction of the microbiome with the innate immune response in chronic wounds. Adv. Exp. Med. Biol. 2012, 946, 55–68. [Google Scholar] [PubMed]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, S.; Longaker, M.T.; Gurtner, G.C. Hypertrophic scar formation following burns and trauma: New approaches to treatment. PLoS Med. 2007, 4, e234. [Google Scholar] [CrossRef] [PubMed]
- Tziotzios, C.; Profyris, C.; Sterling, J. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics part II. Strategies to reduce scar formation after dermatologic procedures. J. Am. Acad. Dermatol. 2012, 66, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wang, G.G.; Li, W.; Jiang, Y.X.; Lu, X.H.; Zhou, P.P. Heme oxygenase-1 promotes delayed wound healing in diabetic rats. J. Diabetes Res. 2016, 2016, 9726503. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, A.A.; Prawez, S.; Leo, M.D.; Kathirvel, K.; Kumar, D.; Tandan, S.K.; Malik, J.K. Pro-healing potential of hemin: An inducer of heme oxygenase-1. Eur. J. Pharmacol. 2010, 645, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound healing effects of curcumin: A short review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Cremers, N.A.; Lundvig, D.M.; van Dalen, S.C.; Schelbergen, R.F.; van Lent, P.L.; Szarek, W.A.; Regan, R.F.; Carels, C.E.; Wagener, F.A. Curcumin-induced heme oxygenase-1 expression prevents H2O2-induced cell death in wild type and heme oxygenase-2 knockout adipose-derived mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 17974–17999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Volk, H.D.; Willis, D.; Abraham, N.G.; Soares, M.P.; Adema, G.J.; Figdor, C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003, 55, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Choi, A.M. Heme oxygenase-1: From bench to bedside. Am. J. Respir. Crit. Care Med. 2005, 172, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Soares, M.P. Coupling heme and iron metabolism via ferritin h chain. Antioxid. Redox. Signal. 2014, 20, 1754–1769. [Google Scholar] [CrossRef] [PubMed]
- Grochot-Przeczek, A.; Lach, R.; Mis, J.; Skrzypek, K.; Gozdecka, M.; Sroczynska, P.; Dubiel, M.; Rutkowski, A.; Kozakowska, M.; Zagorska, A.; et al. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS ONE 2009, 4, e5803. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, A.A.; Leo, M.D.; Gopal, A.; Kant, V.; Tandan, S.K.; Kumar, D. Pro-healing effects of bilirubin in open excision wound model in rats. Int. Wound J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, M.V.; Loiola, R.T.; Rocha, E.L.; Simao, A.F.; Gomes, A.S.; Souza, M.H.; Ribeiro, R.A. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Braz. J. Med. Biol. Res. 2009, 42, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Remote ischaemic preconditioning: Underlying mechanisms and clinical application. Cardiovasc. Res. 2008, 79, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.R.; Chang, K.J.; Chen, C.F.; Tsai, H.W. Transient limb ischemia induces remote preconditioning in liver among rats: The protective role of heme oxygenase-1. Transplantation 2006, 81, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Tapuria, N.; Junnarkar, S.P.; Dutt, N.; Abu-Amara, M.; Fuller, B.; Seifalian, A.M.; Davidson, B.R. Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford) 2009, 11, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Hata, K.; Tanaka, H.; Hirao, H.; Kubota, T.; Okamura, Y.; Iwaisako, K.; Takada, Y.; Uemoto, S. Intestinal ischemic preconditioning ameliorates hepatic ischemia reperfusion injury in rats: Role of heme oxygenase-1 in the second-window of protection. Liver Transpl. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shen, J.; Xiong, X.; Xu, Y.; Zhang, H.; Huang, C.; Tian, Y.; Jiao, C.; Wang, X.; Li, X. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS ONE 2014, 9, e98834. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, M.; Kottenberg, E.; Kleinbongard, P.; Wendt, D.; Gedik, N.; Pasa, S.; Price, V.; Tsagakis, K.; Neuhauser, M.; Peters, J.; et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: A single-centre randomised, double-blind, controlled trial. Lancet 2013, 382, 597–604. [Google Scholar] [CrossRef]
- Saeki, I.; Matsuura, T.; Hayashida, M.; Taguchi, T. Ischemic preconditioning and remote ischemic preconditioning have protective effect against cold ischemia-reperfusion injury of rat small intestine. Pediatr. Surg. Int. 2011, 27, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Mortensen, U.M.; White, P.A.; Kristiansen, S.B.; Schmidt, M.R.; Hoschtitzky, J.A.; Vogel, M.; Sorensen, K.; Redington, A.N.; MacAllister, R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002, 106, 2881–2883. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Hausenloy, D.J. Remote ischemic conditioning: From bench to bedside. Front. Physiol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Wever, K.E.; Masereeuw, R.; Wagener, F.A.; Verweij, V.G.; Peters, J.G.; Pertijs, J.C.; van der Vliet, J.A.; Warle, M.C.; Rongen, G.A. Humoral signalling compounds in remote ischaemic preconditioning of the kidney, a role for the opioid receptor. Nephrol. Dial. Transplant. 2013, 28, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Wever, K.E.; Warle, M.C.; Wagener, F.A.; van der Hoorn, J.W.; Masereeuw, R.; van der Vliet, J.A.; Rongen, G.A. Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: The role of adenosine. Nephrol. Dial. Transplant. 2011, 26, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Jan, W.C.; Chen, C.H.; Tsai, P.S.; Huang, C.J. Limb ischemic preconditioning mitigates lung injury induced by haemorrhagic shock/resuscitation in rats. Resuscitation 2011, 82, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.V.; Dave, K.R.; Perez-Pinzon, M.A. Ischemic preconditioning and clinical scenarios. Curr. Opin. Neurol. 2013, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Depre, C.; Park, J.Y.; Shen, Y.T.; Zhao, X.; Qiu, H.; Yan, L.; Tian, B.; Vatner, S.F.; Vatner, D.E. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am. J. Physiol. Heart. Circ. Physiol. 2010, 299, H752–H762. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.T.; Depre, C.; Yan, L.; Park, J.Y.; Tian, B.; Jain, K.; Chen, L.; Zhang, Y.; Kudej, R.K.; Zhao, X.; et al. Repetitive ischemia by coronary stenosis induces a novel window of ischemic preconditioning. Circulation 2008, 118, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Boston-Griffiths, E.; Yellon, D.M. Cardioprotection during cardiac surgery. Cardiovasc. Res. 2012, 94, 253–265. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, L.; Hong, D.; Wong, M.G.; Badve, S.V.; Rogers, K.; Perkovic, V.; Walsh, M.; Yu, X.; Hillis, G.S.; Gallagher, M.; et al. Effects of ischaemic conditioning on major clinical outcomes in people undergoing invasive procedures: Systematic review and meta-analysis. BMJ 2016, 355, i5599. [Google Scholar] [CrossRef] [PubMed]
- Garratt, K.N.; Whittaker, P.; Przyklenk, K. Remote ischemic conditioning and the long road to clinical translation: Lessons learned from ericca and ripheart. Circ. Res. 2016, 118, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- King, N.D.G.; Smart, N.A. Remote ischaemic pre-conditioning does not affect clinical outcomes following coronary artery bypass grafting. A systematic review and meta-analysis. Clin. Trials Regul. Sci. Cardiol. 2016, 17, 1–8. [Google Scholar] [CrossRef]
- Przyklenk, K. Ischaemic conditioning: Pitfalls on the path to clinical translation. Br. J. Pharmacol. 2015, 172, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Dorresteijn, M.J.; Paine, A.; Zilian, E.; Fenten, M.G.; Frenzel, E.; Janciauskiene, S.; Figueiredo, C.; Eiz-Vesper, B.; Blasczyk, R.; Dekker, D.; et al. Cell-type-specific downregulation of heme oxygenase-1 by lipopolysaccharide via bach1 in primary human mononuclear cells. Free Radic. Biol. Med. 2015, 78, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.; Lorenzen, J.; Kabbani, M.; Herold, C.; Busche, M.; Vogt, P.M.; Knobloch, K. Acute effects of remote ischemic preconditioning on cutaneous microcirculation--a controlled prospective cohort study. BMC Surg. 2011, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Kolbenschlag, J.; Sogorski, A.; Kapalschinski, N.; Harati, K.; Lehnhardt, M.; Daigeler, A.; Hirsch, T.; Goertz, O. Remote ischemic conditioning improves blood flow and oxygen saturation in pedicled and free surgical flaps. Plast. Reconstr. Surg. 2016, 138, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, K.; Asato, H.; Umekawa, K.; Imanishi, M.; Suzuki, A. Value of remote ischaemic preconditioning in rat dorsal skin flaps and clamping time. J. Plast. Surg. Hand. Surg. 2015, 50, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Shaked, G.; Czeiger, D.; Abu Arar, A.; Katz, T.; Harman-Boehm, I.; Sebbag, G. Intermittent cycles of remote ischemic preconditioning augment diabetic foot ulcer healing. Wound Repair Regen. 2015, 23, 191–196. [Google Scholar] [CrossRef]
- Epps, J.A.; Smart, N.A. Remote ischaemic conditioning in the context of type 2 diabetes and neuropathy: The case for repeat application as a novel therapy for lower extremity ulceration. Cardiovasc. Diabetol. 2016, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Contag, P.R.; Hardy, J.; Zhao, H.; Vreman, H.J.; Hajdena-Dawson, M.; Wong, R.J.; Stevenson, D.K.; Contag, C.H. Selection of potential therapeutics based on in vivo spatiotemporal transcription patterns of heme oxygenase-1. J. Molecular Med. 2002, 80, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Kim, J.; Traylor, A.M.; Sanders, P.W.; Balla, J.; Agarwal, A.; Curtis, L.M. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am. J. Physiol. Ren. Physiol. 2011, 300, F254–262. [Google Scholar] [CrossRef] [PubMed]
- Halilovic, A.; Patil, K.A.; Bellner, L.; Marrazzo, G.; Castellano, K.; Cullaro, G.; Dunn, M.W.; Schwartzman, M.L. Knockdown of heme oxygenase-2 impairs corneal epithelial cell wound healing. J. Cell. Physiol. 2011, 226, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation 2008, 117, 231–241. [Google Scholar] [CrossRef]
- Ahanger, A.A.; Prawez, S.; Kumar, D.; Prasad, R.; Amarpal; Tandan, S.K.; Kumar, D. Wound healing activity of carbon monoxide liberated from co-releasing molecule (co-rm). Naunyn Schmiedebergs Arch. Pharmacol. 2011, 384, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Coffman, L.G.; Parsonage, D.; D’Agostino, R., Jr.; Torti, F.M.; Torti, S.V. Regulatory effects of ferritin on angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Granata, R.; Tocchetti, C.G.; Gallo, M.P.; Alloatti, G.; Pagliaro, P. Endogenous cardioprotective agents: Role in pre and postconditioning. Curr. Drug Target. 2015, 16, 843–867. [Google Scholar] [CrossRef]
- Guimaraes Filho, M.A.; Cortez, E.; Garcia-Souza, E.P.; Soares Vde, M.; Moura, A.S.; Carvalho, L.; Maya, M.C.; Pitombo, M.B. Effect of remote ischemic preconditioning in the expression of IL-6 and IL-10 in a rat model of liver ischemia-reperfusion injury. Acta Cir. Bras. 2015, 30, 452–460. [Google Scholar] [CrossRef]
- Chevion, M.; Leibowitz, S.; Aye, N.N.; Novogrodsky, O.; Singer, A.; Avizemer, O.; Bulvik, B.; Konijn, A.M.; Berenshtein, E. Heart protection by ischemic preconditioning: A novel pathway initiated by iron and mediated by ferritin. J. Mol. Cell. Cardiol. 2008, 45, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef] [PubMed]
- Tapuria, N.; Kumar, Y.; Habib, M.M.; Abu Amara, M.; Seifalian, A.M.; Davidson, B.R. Remote ischemic preconditioning: A novel protective method from ischemia reperfusion injury—A review. J. Surg. Res. 2008, 150, 304–330. [Google Scholar] [CrossRef]
- Hanselmann, C.; Mauch, C.; Werner, S. Haem oxygenase-1: A novel player in cutaneous wound repair and psoriasis? Biochem. J. 2001, 353, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kampfer, H.; Kolb, N.; Manderscheid, M.; Wetzler, C.; Pfeilschifter, J.; Frank, S. Macrophage-derived heme-oxygenase-1: Expression, regulation, and possible functions in skin repair. Mol. Med. 2001, 7, 488–498. [Google Scholar] [PubMed]
- Schurmann, C.; Seitz, O.; Klein, C.; Sader, R.; Pfeilschifter, J.; Muhl, H.; Goren, I.; Frank, S. Tight spatial and temporal control in dynamic basal to distal migration of epithelial inflammatory responses and infiltration of cytoprotective macrophages determine healing skin flap transplants in mice. Ann. Surg. 2009, 249, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; van Beurden, H.E.; von den Hoff, J.W.; Adema, G.J.; Figdor, C.G. The heme-heme oxygenase system: A molecular switch in wound healing. Blood 2003, 102, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Holzner, P.A.; Kulemann, B.; Kuesters, S.; Timme, S.; Hoeppner, J.; Hopt, U.T.; Marjanovic, G. Impact of remote ischemic preconditioning on wound healing in small bowel anastomoses. World J. Gastroenterol. 2011, 17, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Colak, T.; Turkmenoglu, O.; Dag, A.; Polat, A.; Comelekoglu, U.; Bagdatoglu, O.; Polat, G.; Kanik, A.; Akca, T.; Aydin, S. The effect of remote ischemic preconditioning on healing of colonic anastomoses. J. Surg. Res. 2007, 143, 200–205. [Google Scholar] [CrossRef]
- Kuntscher, M.V.; Kastell, T.; Engel, H.; Gebhard, M.M.; Heitmann, C.; Germann, G. Late remote ischemic preconditioning in rat muscle and adipocutaneous flap models. Ann. Plastic Surg. 2003, 51, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kuntscher, M.V.; Schirmbeck, E.U.; Menke, H.; Klar, E.; Gebhard, M.M.; Germann, G. Ischemic preconditioning by brief extremity ischemia before flap ischemia in a rat model. Plast. Reconstr. Surg. 2002, 109, 2398–2404. [Google Scholar] [CrossRef]
- Shah, A.A.; Arias, J.E.; Thomson, J.G. The effect of ischemic preconditioning on secondary ischemia in myocutaneous flaps. J. Reconstr. Microsurg. 2009, 25, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Zahir, K.S.; Syed, S.A.; Zink, J.R.; Restifo, R.J.; Thomson, J.G. Ischemic preconditioning improves the survival of skin and myocutaneous flaps in a rat model. Plast. Reconstr. Surg. 1998, 102, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kolh, P. Remote ischaemic pre-conditioning in cardiac surgery: Benefit or not? Eur. Heart J. 2014, 35, 141–143. [Google Scholar] [PubMed]
- Gross, G.J.; Baker, J.E.; Moore, J.; Falck, J.R.; Nithipatikom, K. Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome p450 epoxygenase pathway in canine hearts. Cardiovasc. Drugs Ther. 2011, 25, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.J.; Hsu, A.; Gross, E.R.; Falck, J.R.; Nithipatikom, K. Factors mediating remote preconditioning of trauma in the rat heart: Central role of the cytochrome p450 epoxygenase pathway in mediating infarct size reduction. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.K.; Fan, G.C.; Liao, S.; Zhang, J.M.; Wang, Y.; Weintraub, N.L.; Kranias, E.G.; Schultz, J.E.; Lorenz, J.; Ren, X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase c signaling. Circulation 2009, 120, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Liu, J.; Hu, Y. Cardioprotective effect of remote preconditioning of trauma and remote ischemia preconditioning in a rat model of myocardial ischemia/reperfusion injury. Exp. Ther. Med. 2015, 9, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Scharstuhl, A.; Tyrrell, R.M.; Von den Hoff, J.W.; Jozkowicz, A.; Dulak, J.; Russel, F.G.; Kuijpers-Jagtman, A.M. The heme-heme oxygenase system in wound healing; implications for scar formation. Curr. Drug Target 2010, 11, 1571–1585. [Google Scholar] [CrossRef]
- Lundvig, D.M.; Immenschuh, S.; Wagener, F.A. Heme oxygenase, inflammation, and fibrosis: The good, the bad, and the ugly? Front. Pharmacol. 2012, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Lundvig, D.M.; Scharstuhl, A.; Cremers, N.A.; Pennings, S.W.; te Paske, J.; van Rheden, R.; van Run-van Breda, C.; Regan, R.F.; Russel, F.G.; Carels, C.E.; et al. Delayed cutaneous wound closure in ho-2 deficient mice despite normal ho-1 expression. J. Cell. Mol. Med. 2014, 18, 2488–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auf dem Keller, U.; Kumin, A.; Braun, S.; Werner, S. Reactive oxygen species and their detoxification in healing skin wounds. J. Investig. Dermatol. Symp. Proc. 2006, 11, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Cremers, N.A.; Suttorp, M.; Gerritsen, M.M.; Wong, R.J.; van Run-van Breda, C.; van Dam, G.M.; Brouwer, K.M.; Kuijpers-Jagtman, A.M.; Carels, C.E.; Lundvig, D.M.; et al. Mechanical stress changes the complex interplay between HO-1, inflammation and fibrosis, during excisional wound repair. Front. Med. 2015, 2, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Itoh, K.; Sato, H.; Bannai, S. Oxidative stress-inducible proteins in macrophages. Free Radical Res. 1999, 31, 351–355. [Google Scholar] [CrossRef]
- Dekker, D.; Dorresteijn, M.J.; Pijnenburg, M.; Heemskerk, S.; Rasing-Hoogveld, A.; Burger, D.M.; Wagener, F.A.; Smits, P. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Kihara, Y.; Chayama, K.; et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in gilbert syndrome. Circulation 2012, 126, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Wever, K.E.; Hooijmans, C.R.; Riksen, N.P.; Sterenborg, T.B.; Sena, E.S.; Ritskes-Hoitinga, M.; Warle, M.C. Determinants of the efficacy of cardiac ischemic preconditioning: A systematic review and meta-analysis of animal studies. PLoS ONE 2015, 10, e0142021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wever, K.E.; Wagener, F.A.; Frielink, C.; Boerman, O.C.; Scheffer, G.J.; Allison, A.; Masereeuw, R.; Rongen, G.A. Diannexin protects against renal ischemia reperfusion injury and targets phosphatidylserines in ischemic tissue. PLoS ONE 2011, 6, e24276. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; van Dam, G.M.; Buis, C.I.; Visser, D.S.; Hesselink, J.W.; Schuurs, T.A.; Leuvenink, H.G.; Contag, C.H.; Porte, R.J. Spatiotemporal expression of heme oxygenase-1 detected by in vivo bioluminescence after hepatic ischemia in HO-1/luc mice. Liver Transplant. 2006, 12, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Won, N.H.; Lee, H.W. Remote ischemic preconditioning prevents lipopolysaccharide-induced liver injury through inhibition of NF-κB activation in mice. J. Anesth. 2014, 28, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amara, M.; Yang, S.Y.; Quaglia, A.; Rowley, P.; Tapuria, N.; Seifalian, A.M.; Fuller, B.J.; Davidson, B.R. Effect of remote ischemic preconditioning on liver ischemia/reperfusion injury using a new mouse model. Liver Transplant. 2011, 17, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.P.; Parajuli, N.; Zheng, X.; Becker, L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res. Cardiol. 2012, 107, 277. [Google Scholar] [CrossRef] [PubMed]
- van den Brand, B.T.; Vermeij, E.A.; Waterborg, C.E.; Arntz, O.J.; Kracht, M.; Bennink, M.B.; van den Berg, W.B.; van de Loo, F.A. Intravenous delivery of HIV-based lentiviral vectors preferentially transduces F4/80+ and ly-6c+ cells in spleen, important target cells in autoimmune arthritis. PLoS ONE 2013, 8, e55356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.M.; Mouchaers, K.T.; Schalij, I.; Grunberg, K.; Meijer, G.A.; Vonk-Noordegraaf, A.; van der Laarse, W.J.; Belien, J.A. Rapid quantification of myocardial fibrosis: A new macro-based automated analysis. Cell. Oncol. 2011, 34, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, B.; Winbladh, A.; Bojmar, L.; Sundqvist, T.; Gullstrand, P.; Sandstrom, P. Conventional, but not remote ischemic preconditioning, reduces iNOS transcription in liver ischemia/reperfusion. World J. Gastroenterol. 2014, 20, 9506–9512. [Google Scholar] [PubMed]
- He, X.; Zhao, M.; Bi, X.Y.; Yu, X.J.; Zang, W.J. Delayed preconditioning prevents ischemia/reperfusion-induced endothelial injury in rats: Role of ROS and eNOS. Lab. Investig. 2013, 93, 168–180. [Google Scholar] [CrossRef] [PubMed]





| Aim Experiment | Read Out | Animals (n: ♂/♀) |
|---|---|---|
| Investigate the effects of RIPC on HO-1 promoter activity | HO-1 promoter activity at 1, 6 and 24 h after RIPC treatment | 6: 0/6 |
| Investigate the effects of RIPC on HO-1 gene expression in different organs during time | HO-1 mRNA levels at 0, 1, 6, and 24 h after RIPC treatment | 24: 0/24 (6 per time point) |
| Investigate the effects of RIPC on dermal wound healing | Early (5 min before wounding) and late (24 h before wounding) effects of RIPC on wound healing compared to controls without receiving RIPC treatment (endpoint: day 7) | 6: 4/2 (early RIPC) 6: 4/2 (late RIPC) 8: 4/4 (controls) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremers, N.A.J.; Wever, K.E.; Wong, R.J.; Van Rheden, R.E.M.; Vermeij, E.A.; Van Dam, G.M.; Carels, C.E.; Lundvig, D.M.S.; Wagener, F.A.D.T.G. Effects of Remote Ischemic Preconditioning on Heme Oxygenase-1 Expression and Cutaneous Wound Repair. Int. J. Mol. Sci. 2017, 18, 438. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18020438
Cremers NAJ, Wever KE, Wong RJ, Van Rheden REM, Vermeij EA, Van Dam GM, Carels CE, Lundvig DMS, Wagener FADTG. Effects of Remote Ischemic Preconditioning on Heme Oxygenase-1 Expression and Cutaneous Wound Repair. International Journal of Molecular Sciences. 2017; 18(2):438. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18020438
Chicago/Turabian StyleCremers, Niels A. J., Kimberley E. Wever, Ronald J. Wong, René E. M. Van Rheden, Eline A. Vermeij, Gooitzen M. Van Dam, Carine E. Carels, Ditte M. S. Lundvig, and Frank A. D. T. G. Wagener. 2017. "Effects of Remote Ischemic Preconditioning on Heme Oxygenase-1 Expression and Cutaneous Wound Repair" International Journal of Molecular Sciences 18, no. 2: 438. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18020438