Trauma and Stem Cells: Biology and Potential Therapeutic Implications
Abstract
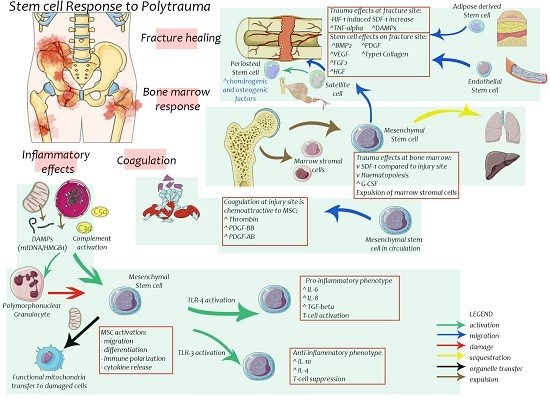
:1. Introduction
2. Stem Cell Biology
3. Effects of Trauma on Stem Cells
4. Stem Cells in Bone Healing
5. Stem Cells in Chondral Healing
6. Stem Cells in Post-Injury Inflammation and Multiple Organ Failure
7. Stem Cells in Wound Healing
8. Stem Cells in Muscle Healing
9. Stem Cells in Angiogenesis
10. Current State of Stem Cell Research
11. Future Directions
Conflicts of Interest
References
- Cameron, P.A.; Gabbe, B.J.; Cooper, D.J.; Walker, T.; Judson, R.; McNeil, J. A statewide system of trauma care in Victoria: Effect on patient survival. Med. J. Aust. 2008, 189, 546–550. [Google Scholar] [PubMed]
- Ursic, C.; Curtis, K.; Zou, Y.; Black, D. Improved trauma patient outcomes after implementation of a dedicated trauma admitting service. Injury 2009, 40, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.; Caldwell, E.; Delprado, A.; Munroe, B. Traumatic injury in Australia and New Zealand. Australas. Emerg. Nurs. J. 2012, 15, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jurkovich, G.J.; Mock, C. Systematic review of trauma system effectiveness based on registry comparisons. J. Trauma 1999, 47 (Suppl. S3), S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Weissman, I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef]
- Li, L.; Xie, T. Stem cell niche: Structure and function. Annu. Rev. Cell Dev. Biol. 2005, 21, 605–631. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; Discipio, R.G.; Khaldoyanidi, S. Mesenchymal stem cells and their microenvironment. Front. Biosci. 2011, 16, 2271–2288. [Google Scholar] [CrossRef]
- Wilson, A.; Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006, 6, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Blau, H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature 2010, 465, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Hochedlinger, K.; Jaenisch, R. Nuclear reprogramming and pluripotency. Nature 2006, 441, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Wiegner, R.; Lampl, L.; Brenner, R.E. Mesenchymal Stem Cells after Polytrauma: Actor and Target. Stem Cells Int. 2016, 2016, 6289825. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Chung Kuo Yao Li Hsueh Pao 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fu, X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012, 348, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.M.; Weissman, I.L.; Tsukamoto, A.S.; Buckle, A.M.; Peault, B. Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl. Acad. Sci. USA 1992, 89, 2804–2808. [Google Scholar] [CrossRef] [PubMed]
- Xynos, A.; Corbella, P.; Belmonte, N.; Zini, R.; Manfredini, R.; Ferrari, G. Bone marrow-derived hematopoietic cells undergo myogenic differentiation following a Pax-7 independent pathway. Stem Cells 2010, 28, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Pelus, L.M. Mobilization of hematopoietic stem cells from the bone marrow niche to the blood compartment. Stem Cell Res. Ther. 2011, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Meric, A.; Yenigun, A.; Yenigun, V.B.; Dogan, R.; Ozturan, O. Comparison of chondrocytes produced from adipose tissue-derived stem cells and cartilage tissue. J. Craniofac. Surg. 2013, 24, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Yang, M.; Liang, Y.; Li, Q. Adipose tissue-derived stem cells (ADSCs) transplantation promotes regeneration of expanded skin using a tissue expansion model. Wound Repair Regen. 2013, 21, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Molcanyi, M.; Riess, P.; Bentz, K.; Maegele, M.; Hescheler, J.; Schafke, B.; Trapp, T.; Neugebauer, E.; Klug, N.; Schafer, U. Trauma-associated inflammatory response impairs embryonic stem cell survival and integration after implantation into injured rat brain. J. Neurotrauma 2007, 24, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.H.; Wang, Y.; Lu, Z.M.; Zhou, H.; Xue, X.C.; Bi, J.W.; Ma, L.Y.; Fang, G.E. The change and effect of endothelial progenitor cells in pig with multiple organ dysfunction syndromes. Crit. Care 2009, 13, R118. [Google Scholar] [CrossRef] [PubMed]
- Livingston, D.H.; Anjaria, D.; Wu, J.; Hauser, C.J.; Chang, V.; Deitch, E.A.; Rameshwar, P. Bone marrow failure following severe injury in humans. Ann. Surg. 2003, 238, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Rennert, R.C.; Sorkin, M.; Garg, R.K.; Gurtner, G.C. Stem cell recruitment after injury: Lessons for regenerative medicine. Regen Med. 2012, 7, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Gul-Uludag, H.; Xu, P.; Chen, J.; Janowska-Wieczorek, A. CXCR4 transfection of cord blood mesenchymal stromal cells with the use of cationic liposome enhances their migration toward stromal cell-derived factor-1. Cytotherapy 2013, 15, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. BioMed Res. Int. 2013, 2013, 561098. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Han, B.; Cai, S.; Lei, Y.; Sun, T.; Sheng, Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen. 2009, 17, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Nishimura, M.; Sekiya, K.; Suehiro, F.; Kanawa, M.; Nikawa, H.; Hamada, T.; Kato, Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007, 16, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, L.A.; Barfield, W.R.; Kokko, K.P.; Liles, L.L.; Wind, T.; Green, J.; Giannoudis, P.V. Randomized prospective clinical trial comparing reamer irrigator aspirator (RIA) to standard reaming (SR) in both minimally injured and multiply injured patients with closed femoral shaft fractures treated with reamed intramedullary nailing (IMN). Injury 2010, 41 (Suppl. S2), S94–S98. [Google Scholar] [CrossRef]
- Pape, H.C.; Grimme, K.; Van Griensven, M.; Sott, A.H.; Giannoudis, P.; Morley, J.; Roise, O.; Ellingsen, E.; Hildebrand, F.; Wiese, B.; et al. Impact of intramedullary instrumentation versus damage control for femoral fractures on immunoinflammatory parameters: Prospective randomized analysis by the EPOFF Study Group. J. Trauma 2003, 55, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, N.M.; Chandler, W.L. Thrombin generation in trauma patients. Transfusion 2009, 49, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Spies, V.; Mehling, I.; Gruszka, D.; Rommens, P.M.; Hofmann, A. Mobilization of CD34+-progenitor cells in patients with severe trauma. PLoS ONE 2014, 9, e97369. [Google Scholar] [CrossRef] [PubMed]
- Henrich, D.; Zimmer, S.; Seebach, C.; Frank, J.; Barker, J.; Marzi, I. Trauma-activated polymorphonucleated leukocytes damage endothelial progenitor cells: Probable role of CD11b/CD18-CD54 interaction and release of reactive oxygen species. Shock 2011, 36, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Fernandez, R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J. Hypertens. 2009, 27, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, T.G.; Heymes, C.; You, D.; Blanc-Brude, O.; Mees, B.; Waeckel, L.; Duriez, M.; Vilar, J.; Brandes, R.P.; Levy, B.I.; et al. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am. J. Pathol. 2006, 169, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Koenen, P.; Spanholtz, T.A.; Maegele, M.; Sturmer, E.; Brockamp, T.; Neugebauer, E.; Thamm, O.C. Acute and chronic wound fluids inversely influence adipose-derived stem cell function: Molecular insights into impaired wound healing. Int. Wound J. 2015, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rüedi, T.P.; Murphy, W.M. AO Principles of Fracture Management; Thieme: New York, NY, USA, 2000. [Google Scholar]
- Tawonsawatruk, T.; Kelly, M.; Simpson, H. Evaluation of native mesenchymal stem cells from bone marrow and local tissue in an atrophic nonunion model. Tissue Eng. Part C Methods 2014, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Toupadakis, C.A.; Granick, J.L.; Sagy, M.; Wong, A.; Ghassemi, E.; Chung, D.J.; Borjesson, D.L.; Yellowley, C.E. Mobilization of endogenous stem cell populations enhances fracture healing in a murine femoral fracture model. Cytotherapy 2013, 15, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Granero-Molto, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Atesok, K.; Li, R.; Stewart, D.J.; Schemitsch, E.H. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J. Orthop. Res. 2010, 28, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kawamoto, A.; Kuroda, R.; Ishikawa, M.; Mifune, Y.; Iwasaki, H.; Miwa, M.; Horii, M.; Hayashi, S.; Oyamada, A.; et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am. J. Pathol. 2006, 169, 1440–1457. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, Y.H.; Sung, L.Y.; Chen, C.L.; Lin, S.Y.; Li, K.C.; Yen, T.C.; Lin, K.J.; Hu, Y.C. Long-term tracking of segmental bone healing mediated by genetically engineered adipose-derived stem cells: Focuses on bone remodeling and potential side effects. Tissue Eng. Part A 2014, 20, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, R.; Yang, F.; Lieu, S.; Julien, A.; Perry, J.; Pereira, C.; Relaix, F.; Miclau, T.; Marcucio, R.; Colnot, C. Role of muscle stem cells during skeletal regeneration. Stem Cells 2015, 33, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Haudenschild, A.K.; Hsieh, A.H.; Kapila, S.; Lotz, J.C. Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann. Biomed. Eng. 2009, 37, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kearney, E.M.; Farrell, E.; Prendergast, P.J.; Campbell, V.A. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann. Biomed. Eng. 2010, 38, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.N.; Yoon, H.H.; Seo, Y.K.; Park, J.K. Effect of mechanical stimulation on the differentiation of cord stem cells. Connect. Tissue Res. 2012, 53, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kusuyama, J.; Bandow, K.; Shamoto, M.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J. Biol. Chem. 2014, 289, 10330–10344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Buckley, C.T.; Almeida, H.V.; Mulhall, K.J.; Kelly, D.J. Infrapatellar fat pad-derived stem cells maintain their chondrogenic capacity in disease and can be used to engineer cartilaginous grafts of clinically relevant dimensions. Tissue Eng. Part A 2014, 20, 3050–3062. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.Q.; Wang, R.; Ling, S.K.; Wan, Y.M.; Li, Y.H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 2007, 40, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Endo, N.; Yamagiwa, H.; Tanizawa, T.; Takahashi, H.E. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J. Bone Miner. Metab. 1999, 17, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rajaratnam, J.H.; Denton, J.; Hoyland, J.A.; Byers, R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Leucht, P.; Jiang, J.; Cheng, D.; Liu, B.; Dhamdhere, G.; Fang, M.Y.; Monica, S.D.; Urena, J.J.; Cole, W.; Smith, L.R.; et al. Wnt3a reestablishes osteogenic capacity to bone grafts from aged animals. J. Bone Jt. Surg. Am. 2013, 95, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Shenaq, D.S.; Rastegar, F.; Petkovic, D.; Zhang, B.Q.; He, B.C.; Chen, L.; Zuo, G.W.; Luo, Q.; Shi, Q.; Wagner, E.R.; et al. Mesenchymal Progenitor Cells and Their Orthopedic Applications: Forging a Path towards Clinical Trials. Stem Cells Int. 2010, 2010, 519028. [Google Scholar] [CrossRef] [PubMed]
- Liebergall, M.; Schroeder, J.; Mosheiff, R.; Gazit, Z.; Yoram, Z.; Rasooly, L.; Daskal, A.; Khoury, A.; Weil, Y.; Beyth, S. Stem cell-based therapy for prevention of delayed fracture union: A randomized and prospective preliminary study. Mol. Ther. 2013, 21, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Matsumoto, T.; Niikura, T.; Kawakami, Y.; Fukui, T.; Lee, S.Y.; Mifune, Y.; Kawamata, S.; Fukushima, M.; Asahara, T.; et al. Local transplantation of granulocyte colony stimulating factor-mobilized CD34+ cells for patients with femoral and tibial nonunion: Pilot clinical trial. Stem Cells Transl. 2014, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kurth, T.B.; Dell’accio, F.; Crouch, V.; Augello, A.; Sharpe, P.T.; De Bari, C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011, 63, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Plaas, A.; Velasco, J.; Gorski, D.J.; Li, J.; Cole, A.; Christopherson, K.; Sandy, J.D. The relationship between fibrogenic TGFbeta1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Anemaet, W.; Diaz, M.A.; Buchanan, S.; Tortorella, M.; Malfait, A.M.; Mikecz, K.; Sandy, J.D.; Plaas, A. Knockout of ADAMTS5 does not eliminate cartilage aggrecanase activity but abrogates joint fibrosis and promotes cartilage aggrecan deposition in murine osteoarthritis models. J. Orthop. Res. 2011, 29, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Li, J.; DiPietro, L.; Stepp, M.A.; Sandy, J.D.; Plaas, A. Adamts5 Deletion Blocks Murine Dermal Repair through CD44-mediated Aggrecan Accumulation and Modulation of Transforming Growth Factor β1 (TGFβ1) Signaling. J. Biol. Chem. 2011, 286, 26016–26027. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Wu, C.L.; Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transpl. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.; Jablonski, C.L.; Leonard, C.A.; Dunn, J.F.; Raharjo, E.; Matyas, J.R.; Biernaskie, J.; Krawetz, R.J. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci. Rep. 2016, 6, 23076. [Google Scholar] [CrossRef] [PubMed]
- Honczarenko, M.; Le, Y.; Swierkowski, M.; Ghiran, I.; Glodek, A.M.; Silberstein, L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006, 24, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.Y.; Anz, A.; Siew-Yoke Jee, C.; Merican, S.; Ching-Soong Ng, R.; Roohi, S.A.; Ragavanaidu, K. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial. Arthroscopy 2013, 29, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentis, J.; Sanchez, A.; Garcia-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results. Transplantation 2014, 97, e66–e68. [Google Scholar] [CrossRef] [PubMed]
- Vangsness, C.T.; Farr, J.; Boyd, J.; Dellaero, D.T.; Mills, C.R.; LeRoux-Williams, M. Adult Human Mesenchymal Stem Cells Delivered via Intra-Articular Injection to the Knee Following Partial Medial Meniscectomy. A Randomized, Double-Blind, Controlled Study. J. Bone Jt. Surg. Am. 2014, 96, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wyles, C.C.; Houdek, M.T.; Behfar, A.; Sierra, R.J. Mesenchymal stem cell therapy for osteoarthritis: Current perspectives. Stem Cells Cloning 2015, 8, 117–124. [Google Scholar] [PubMed]
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. BMC Musculoskelet. Disord. 2016, 17, 1016–1085. [Google Scholar] [CrossRef] [PubMed]
- Reissis, D.; Tang, Q.O.; Cooper, N.C.; Carasco, C.F.; Gamie, Z.; Mantalaris, A.; Tsiridis, E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin. Biol. Ther. 2016, 16, 535–557. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed]
- Pevsner-Fischer, M.; Morad, V.; Cohen-Sfady, M.; Rousso-Noori, L.; Zanin-Zhorov, A.; Cohen, S.; Cohen, I.R.; Zipori, D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 2007, 109, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, M.; Yuan, Y.; Wang, Z.Z.; Jiang, H.; Chen, T. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem. Biophys. Res. Commun. 2014, 448, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Nurmenniemi, S.; Kuvaja, P.; Lehtonen, S.; Tiuraniemi, S.; Alahuhta, I.; Mattila, R.K.; Risteli, J.; Salo, T.; Selander, K.S.; Nyberg, P.; et al. Toll-like receptor 9 ligands enhance mesenchymal stem cell invasion and expression of matrix metalloprotease-13. Exp. Cell Res. 2010, 316, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.J.; Bigland, M.; White, A.E.; Hardy, B.M.; Lott, N.; Smith, D.W.; Balogh, Z.J. Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma surgery. J. Trauma Acute Care Surg. 2015, 78, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- IIslam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Tianhang, L.; Bo, W.; Zhengmao, L.; Tao, P.; Hong, Z.; Xuchao, X.; Jianwei, B.; Hui, Z.; Guoen, F. Autologous transplantation of endothelial progenitor cells to prevent multiple organ dysfunction syndromes in pig. J. Trauma Acute Care Surg. 2013, 74, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.M. The Role of Plasma G-CSF and Bone Marrow Dysfunction after Severe Trauma. J. Am. Coll. Surg. 2013, 216, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Szyper-Kravitz, M.; Nagler, A.; Lahav, M.; Peled, A.; Habler, L.; Ponomaryov, T.; Taichman, R.S.; Arenzana-Seisdedos, F.; Fujii, N.; et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002, 3, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.V.; Bible, L.E.; Livingston, D.H.; Mohr, A.M.; Sifri, Z.C. Mesenchymal Stem Cells Reverse Bone Marrow Dysfunction Following Injury and Stress. J. Trauma Acute Care Surg. 2015, 79, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.V.; Bible, L.E.; Livingston, D.H.; Mohr, A.M.; Sifri, Z.C. Mesenchymal stem cells reverse trauma and hemorrhagic shock-induced bone marrow dysfunction. J. Surg. Res. 2015, 199, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Masterson, C.; Devaney, J.; Barry, F.; Elliman, S.; O’Brien, T.; O’Toole, D.; Curley, G.F.; Laffey, J.G. Therapeutic efficacy of human mesenchymal stromal cells in the repair of established ventilator-induced lung injury in the rat. Anesthesiology 2015, 122, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Maron-Gutierrez, T.; Silva, J.D.; Asensi, K.D.; Bakker-Abreu, I.; Shan, Y.; Diaz, B.L.; Goldenberg, R.C.; Mei, S.H.; Stewart, D.J.; Morales, M.M.; et al. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit. Care Med. 2013, 41, e319–e333. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Masterson, C.; Devaney, J.; Barry, F.; Elliman, S.; O’Brien, T.; D, O.T.; Curley, G.F.; Laffey, J.G.; Lee, J.W.; et al. Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome: A Light at the End of the Tunnel?: Optimizing Therapeutic Potential of Human Mesenchymal Stromal Cells to Enhance Repair following Ventilator Induced Lung Injury in the Rat. Anesthesiology 2015, 122, 238–240. [Google Scholar]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef] [PubMed]
- Ogliari, K.S.; Marinowic, D.; Brum, D.E.; Loth, F. Stem cells in dermatology. Anais Brasileiros de Dermatologia 2014, 89, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Lorenz, K.; Richter, A.; Scheffler, K.; Kern, L.; Ebert, S.; Giri, S.; Behrens, M.; Dornseifer, U.; Macchiarini, P.; et al. Interactive role of trauma cytokines and erythropoietin and their therapeutic potential for acute and chronic wounds. Rejuvenation Res. 2011, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Niyaz, M.; Gurpinar, O.A.; Oktar, G.L.; Gunaydin, S.; Onur, M.A.; Ozsin, K.K.; Yener, A. Effects of VEGF and MSCs on vascular regeneration in a trauma model in rats. Wound Repair Regen. 2015, 23, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- De Moya, M.A.; Dunham, M.; Inaba, K.; Bahouth, H.; Alam, H.B.; Sultan, B.; Namias, N. Long-term outcome of acellular dermal matrix when used for large traumatic open abdomen. J. Trauma 2008, 65, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhang, G.; Yang, D.; Liu, T.; Liu, D.; Xu, J.; Zhang, J. Targeted delivery of adipose-derived stem cells via acellular dermal matrix enhances wound repair in diabetic rats. J. Tissue Eng. Regen. Med. 2015, 9, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L.; Paternostro, F.; Tani, A.; Mirabella, C.; Quattrini Li, A.; Nosi, D.; D’Asta, F.; Saccardi, R.; Mazzanti, B.; Lo Russo, G.; et al. MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound Repair Regen. 2015, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Joint Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Foster, W.; Deasy, B.M.; Chan, Y.; Prisk, V.; Tang, Y.; Cummins, J.; Huard, J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. Am. J. Pathol. 2004, 164, 1007–1019. [Google Scholar] [CrossRef]
- Li, Y.; Huard, J. Differentiation of Muscle-Derived Cells into Myofibroblasts in Injured Skeletal Muscle. Am. J. Pathol. 2002, 161, 895–907. [Google Scholar] [CrossRef]
- Fu, X.; Wang, H.; Hu, P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015, 72, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Lucia, A.; Gómez-Gallego, F.; Esteve-Lanao, J.; Pérez-Martínez, A.; Foster, C.; Andreu, A.L.; Martin, M.A.; Madero, L.; Arenas, J.; et al. Mobilisation of mesenchymal cells into blood in response to skeletal muscle injury. Br. J. Sports Med. 2006, 40, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 2003, 278, 12601–12604. [Google Scholar] [CrossRef] [PubMed]
- Montarras, D.; L’Honore, A.; Buckingham, M. Lying low but ready for action: The quiescent muscle satellite cell. FEBS J. 2013, 280, 4036–4050. [Google Scholar] [CrossRef] [PubMed]
- Von Roth, P.; Duda, G.N.; Radojewski, P.; Preininger, B.; Perka, C.; Winkler, T. Mesenchymal stem cell therapy following muscle trauma leads to improved muscular regeneration in both male and female rats. Gend Med. 2012, 9, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; von Roth, P.; Radojewski, P.; Urbanski, A.; Hahn, S.; Preininger, B.; Duda, G.N.; Perka, C. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J. Tissue Eng. Regen. Med. 2012, 6 (Suppl. S3), s60–s67. [Google Scholar] [CrossRef] [PubMed]
- Ii, M.; Nishimura, H.; Iwakura, A.; Wecker, A.; Eaton, E.; Asahara, T.; Losordo, D.W. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 2005, 111, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Rafii, S.; Wu, M.H.; Wijelath, E.S.; Yu, C.; Ishida, A.; Fujita, Y.; Kothari, S.; Mohle, R.; Sauvage, L.R.; et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998, 92, 362–367. [Google Scholar] [PubMed]
- Crosby, J.R.; Kaminski, W.E.; Schatteman, G.; Martin, P.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ. Res. 2000, 87, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [PubMed]
- Urbich, C.; Aicher, A.; Heeschen, C.; Dernbach, E.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell. Cardiol. 2005, 39, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Miranville, A.; Heeschen, C.; Sengenes, C.; Curat, C.A.; Busse, R.; Bouloumie, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Ji, C.; Wei, Z.; Chen, H.; Zhang, J. The Therapeutic Role of VEGF-Expressing Muscle-Derived Stem Cells in Acute Penile Cavernosal Injury. J. Sex. Med. 2012, 9, 1988–2000. [Google Scholar] [CrossRef] [PubMed]
- Federman, D.G.; Ladiiznski, B.; Dardik, A.; Kelly, M.; Shapshak, D.; Ueno, C.M.; Mostow, E.N.; Richmond, N.A.; Hopf, H.W. Wound healing society 2014 update on guidelines for arterial ulcers. Wound Repair Regen. 2016, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 2016, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Lee DE, A.N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Burd, A. An update review of stem cell applications in burns and wound care. Indian J. Plast. Surg. 2012, 45, 229–236. [Google Scholar] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurairajah, K.; Broadhead, M.L.; Balogh, Z.J. Trauma and Stem Cells: Biology and Potential Therapeutic Implications. Int. J. Mol. Sci. 2017, 18, 577. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030577
Thurairajah K, Broadhead ML, Balogh ZJ. Trauma and Stem Cells: Biology and Potential Therapeutic Implications. International Journal of Molecular Sciences. 2017; 18(3):577. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030577
Chicago/Turabian StyleThurairajah, Kabilan, Matthew L. Broadhead, and Zsolt J. Balogh. 2017. "Trauma and Stem Cells: Biology and Potential Therapeutic Implications" International Journal of Molecular Sciences 18, no. 3: 577. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030577