ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reductions in TrkA Receptor Levels
Abstract
:1. Introduction
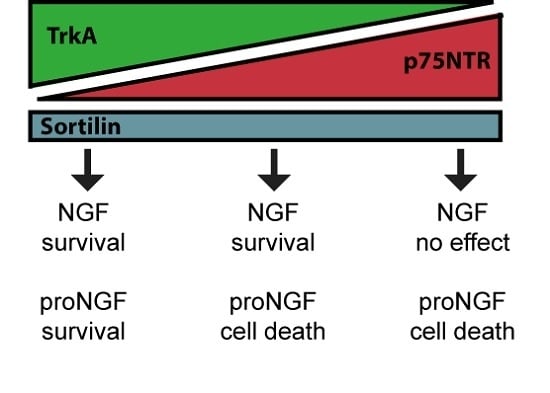
2. Results
2.1. Nerve Growth Factor (NGF) and ProNGF Support Cell Survival Similarly in Primed PC12 Cells
2.2. TrkA Knockdown Has No Effect on Cells Treated with Mature NGF but Increases Cell Death in Response to ProNGF
2.3. ProNGF, but Not NGF, Induces Cell Death in Mutagenized PC12s That Lack TrkA
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Neurotrophins
4.3. Western Blotting
4.4. TrkA Knockdown Bioassay
4.5. PC12nnr5 Bioassay
4.6. Analysis of Cell Death
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EDTA | Ethylene diamine tetra acetic acid |
| ERK | Extracellular signal-regulated kinase |
| Hoechst | Bis benzimide H33342 trihydrochloride |
| NGF | Nerve growth factor |
| p75NTR | Pan-neurotrophin receptor |
| PBS | Phosphate buffered saline |
| PC12 | Rat pheochromocytoma |
| PI3K | Phosphatidylinositol-3-kinase |
| proNGF | Precursor to nerve growth factor |
| proNGF(R-1G) | Cleavage-resistant proNGF |
| RPMI | Roswell Park Memorial Institute medium |
| SCG | Superior cervical ganglion |
| siRNA | Small interfering RNA |
| TrkA | Tropomyosin-related kinase A |
| WT-NGF | Cleavable, wild type proNGF |
References
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Pareek, S.; Savaria, D.; Hamelin, J.; Goulet, B.; Laliberte, J.; Lazure, C.; Chrétien, M.; Murphy, R.A. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem. J. 1996, 314, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Cuello, A.C. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. USA 2006, 103, 6735–6740. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M.; Yu, G.; Michalski, B.; Mathew, S.; Colquhoun, A.; Ross, G.M.; Coughlin, M.D. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J. Neurochem. 2004, 89, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Clewes, O.; Fahey, M.S.; Tyler, S.J.; Watson, J.J.; Seok, H.; Catania, C.; Cho, K.; Dawbarn, D.; Allen, S.J. Human ProNGF: Biological effects and binding profiles at TrkA, P75NTR and sortilin. J. Neurochem. 2008, 107, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M.; Michalski, B.; Xu, B.; Coughlin, M.D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell Neurosci. 2001, 18, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Crowder, R.J.; Freeman, R.S. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 1998, 18, 2933–2943. [Google Scholar] [PubMed]
- Hetman, M.; Kanning, K.; Cavanaugh, J.E.; Xia, Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem. 1999, 274, 22569–22580. [Google Scholar] [CrossRef] [PubMed]
- Cowley, S.; Paterson, H.; Kemp, P.; Marshall, C.J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 1994, 77, 841–852. [Google Scholar] [CrossRef]
- Roux, P.P.; Barker, P.A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002, 67, 203–233. [Google Scholar] [CrossRef]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Lee, K.F.; Jaenisch, R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron 1993, 11, 565–574. [Google Scholar] [CrossRef]
- Mahadeo, D.; Kaplan, L.; Chao, M.V.; Hempstead, B.L. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J. Biol. Chem. 1994, 269, 6884–6891. [Google Scholar] [PubMed]
- Bibel, M.; Hoppe, E.; Barde, Y.A. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999, 18, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Hamanoue, M.; Middleton, G.; Wyatt, S.; Jaffray, E.; Hay, R.T.; Davies, A.M. p75-mediated NF-κB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol. Cell Neurosci. 1999, 14, 28–40. [Google Scholar] [CrossRef] [PubMed]
- De Nadai, T.; Marchetti, L.; di Rienzo, C.; Calvello, M.; Signore, G.; di Matteo, P.; Gobbo, F.; Turturro, S.; Meucci, S.; Viegi, A.; et al. Precursor and mature NGF live tracking: One versus many at a time in the axons. Sci. Rep. 2016, 6, 20272. [Google Scholar] [CrossRef]
- Barrett, G.L.; Bartlett, P.F. The p75 nerve growth factor receptor mediates survival or death depending on the stage of sensory neuron development. Proc. Natl. Acad. Sci. USA 1994, 91, 6501–6505. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Casaccia-Bonnefil, P.; Carter, B.; Chao, M.V. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998, 18, 3273–3281. [Google Scholar] [PubMed]
- Bhakar, A.L.; Howell, J.L.; Paul, C.E.; Salehi, A.H.; Becker, E.B.; Said, F.; Bonni, A.; Barker, P.A. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J. Neurosci. 2003, 23, 11373–11381. [Google Scholar] [PubMed]
- Masoudi, R.; Ioannou, M.S.; Coughlin, M.D.; Pagadala, P.; Neet, K.E.; Clewes, O.; Allen, S.J.; Dawbarn, D.; Fahnestock, M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J. Biol. Chem. 2009, 284, 18424–18433. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, J.; Ceni, C.; Pagdala, P.C.; Forgie, A.; Neet, K.E.; Barker, P.A. Proneurotrophins require endocytosis and intracellular proteolysis to induce TrkA activation. J. Biol. Chem. 2008, 283, 12709–12716. [Google Scholar] [CrossRef] [PubMed]
- Green, S.H.; Rydel, R.E.; Connolly, J.L.; Greene, L.A. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J. Cell Biol. 1986, 102, 830–843. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.I.; Meakin, S.O. Deletions in the extracellular domain of rat trkA lead to an altered differentiative phenotype in neurotrophin responsive cells. Mol. Cell Neurosci. 1996, 7, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, P.C.; Dvorak, L.A.; Neet, K.E. Construction of a mutated pro-nerve growth factor resistant to degradation and suitable for biophysical and cellular utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 17939–17943. [Google Scholar] [CrossRef] [PubMed]
- Loeb, D.M.; Greene, L.A. Transfection with trk restores “slow” NGF binding, efficient NGF uptake, and multiple NGF responses to NGF-nonresponsive PC12 cell mutants. J. Neurosci. 1993, 13, 2919–2929. [Google Scholar] [PubMed]
- Taglialatela, G.; Hibbert, C.J.; Hutton, L.A.; Werrbach-Perez, K.; Perez-Polo, J.R. Suppression of p140trkA does not abolish nerve growth factor-mediated rescue of serum-free PC12 cells. J. Neurochem. 1996, 66, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, S.; Davies, A.M. Regulation of nerve growth factor receptor gene expression in sympathetic neurons during development. J. Cell Biol. 1995, 130, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawi, R.; Hafner, A.; Chun, S.; Raza, S.; Crutcher, K.; Thrasivoulou, C.; Simons, P.; Cowen, T. ProNGF, sortilin, and age-related neurodegeneration. Ann. N. Y. Acad. Sci. 2007, 1119, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.; Giehl, K.; Nyengaard, J.R.; Teng, K.; Lioubinski, O.; Sjoegaard, S.S.; Breiderhoff, T.; Gotthardt, M.; Lin, F.; Eilers, A.; et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat. Neurosci. 2007, 10, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Counts, S.E.; Mufson, E.J. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J. Neuropathol. Exp. Neurol. 2005, 64, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Wuu, J.; Counts, S.E.; Nykjaer, A. Preservation of cortical sortilin protein levels in MCI and Alzheimer’s disease. Neurosci. Lett. 2010, 471, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M.; Scott, S.A.; Jette, N.; Weingartner, J.A.; Crutcher, K.A. Nerve growth factor mRNA and protein levels measured in the same tissue from normal and Alzheimer’s disease parietal cortex. Brain Res. Mol. Brain Res. 1996, 42, 175–178. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J. Neuropathol. Exp. Neurol. 2004, 63, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.S.; Harrington, A.W.; Lee, R.; Kim, J.Y.; Boyce, S.L.; Longo, F.M.; Bresnahan, J.C.; Hempstead, B.L.; Yoon, S.O. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 2002, 36, 375–386. [Google Scholar] [CrossRef]
- Tep, C.; Lim, T.H.; Ko, P.O.; Getahun, S.; Ryu, J.C.; Goettl, V.M.; Massa, S.M.; Basso, M.; Longo, F.M.; Yoon, S.O. Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J. Neurosci. 2013, 33, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Roselli, S.; Demont, Y.; Pundavela, J.; Choquet, G.; Leissner, P.; Oldmeadow, C.; Attia, J.; Walker, M.M.; Hondermarck, H. ProNGF is a potential diagnostic biomarker for thyroid cancer. Oncotarget 2016, 7, 28488–28497. [Google Scholar] [CrossRef] [PubMed]
- Pundavela, J.; Demont, Y.; Jobling, P.; Lincz, L.F.; Roselli, S.; Thorne, R.F.; Bond, D.; Bradshaw, R.A.; Walker, M.M.; Hondermarck, H. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 2014, 184, 3156–3162. [Google Scholar] [CrossRef] [PubMed]
- Goda, M.; Takatori, S.; Atagi, S.; Hashikawa-Hobara, N.; Kawasaki, H.; Cantarella, G.; Lempereur, L.; Presta, M.; Ribatti, D.; Lombardo, G.; et al. Nerve growth factor facilitates perivascular innervation in neovasculatures of mice. J. Pharmacol. Sci. 2016, 131, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve-cancer cell cross-talk: A novel promoter of tumor progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Meignan, S.; Adriaenssens, E.; Foveau, B.; Vanhecke, E.; Romon, R.; Toillon, R.A.; Oxombre, B.; Hondermarck, H.; Le Bourhis, X. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene 2009, 28, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 2013, 12, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Demont, Y.; Corbet, C.; Page, A.; Ataman-Önal, Y.; Choquet-Kastylevsky, G.; Fliniaux, I.; Le Bourhis, X.; Toillon, R.A.; Bradshaw, R.A.; Hondermarck, H. Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J. Biol. Chem. 2012, 287, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Marconi, A.; Lotti, R.; Dallaglio, K.; French, L.E.; Hempstead, B.L.; Pincelli, C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J. Investig. Dermatol. 2008, 128, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Reich, R.; Lazarovici, P.; Ann Flørenes, V.; Nielsen, S.; Nesland, J.M. Altered expression and activation of the nerve growth factor receptors TrkA and p75 provide the first evidence of tumor progression to effusion in breast carcinoma. Breast Cancer Res. Treat. 2004, 83, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mobley, W.C.; Schenker, A.; Shooter, E.M. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry 1976, 15, 5543–5552. [Google Scholar] [CrossRef] [PubMed]
- Bumeister, R.; Rosse, C.; Anselmo, A.; Camonis, J.; White, M.A. CNK2 couples NGF signal propagation to multiple regulatory cascades driving cell differentiation. Curr. Biol. 2004, 14, 439–445. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannou, M.S.; Fahnestock, M. ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reductions in TrkA Receptor Levels. Int. J. Mol. Sci. 2017, 18, 599. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030599
Ioannou MS, Fahnestock M. ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reductions in TrkA Receptor Levels. International Journal of Molecular Sciences. 2017; 18(3):599. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030599
Chicago/Turabian StyleIoannou, Maria S., and Margaret Fahnestock. 2017. "ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reductions in TrkA Receptor Levels" International Journal of Molecular Sciences 18, no. 3: 599. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030599