Nrf2 Regulates the Risk of a Diesel Exhaust Inhalation-Induced Immune Response during Bleomycin Lung Injury and Fibrosis in Mice
Abstract
:1. Introduction
2. Results
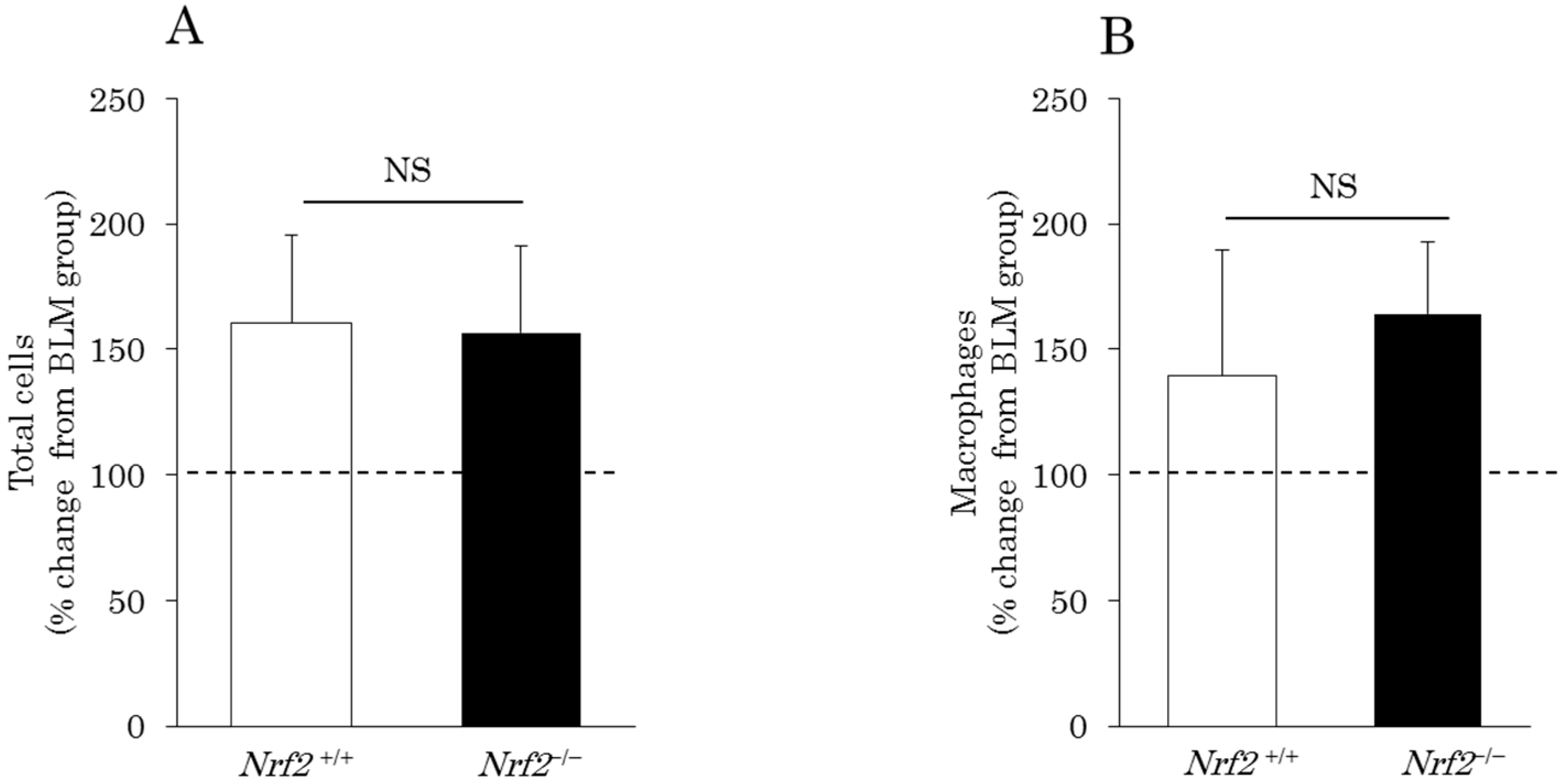
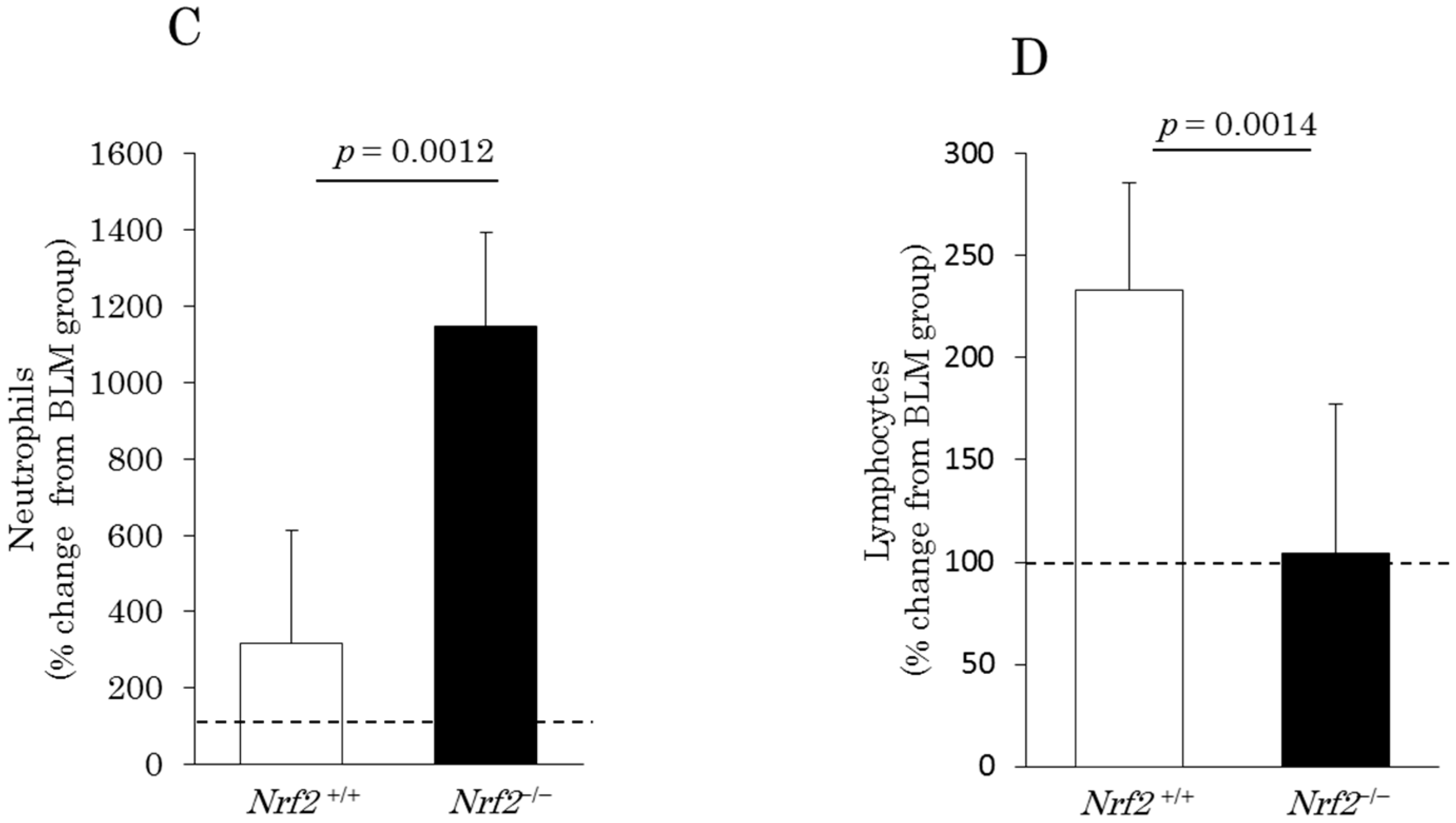
2.1. Differential Cell Counts in Bronchoalveolar Lavage (BAL) Fluid (BALF)
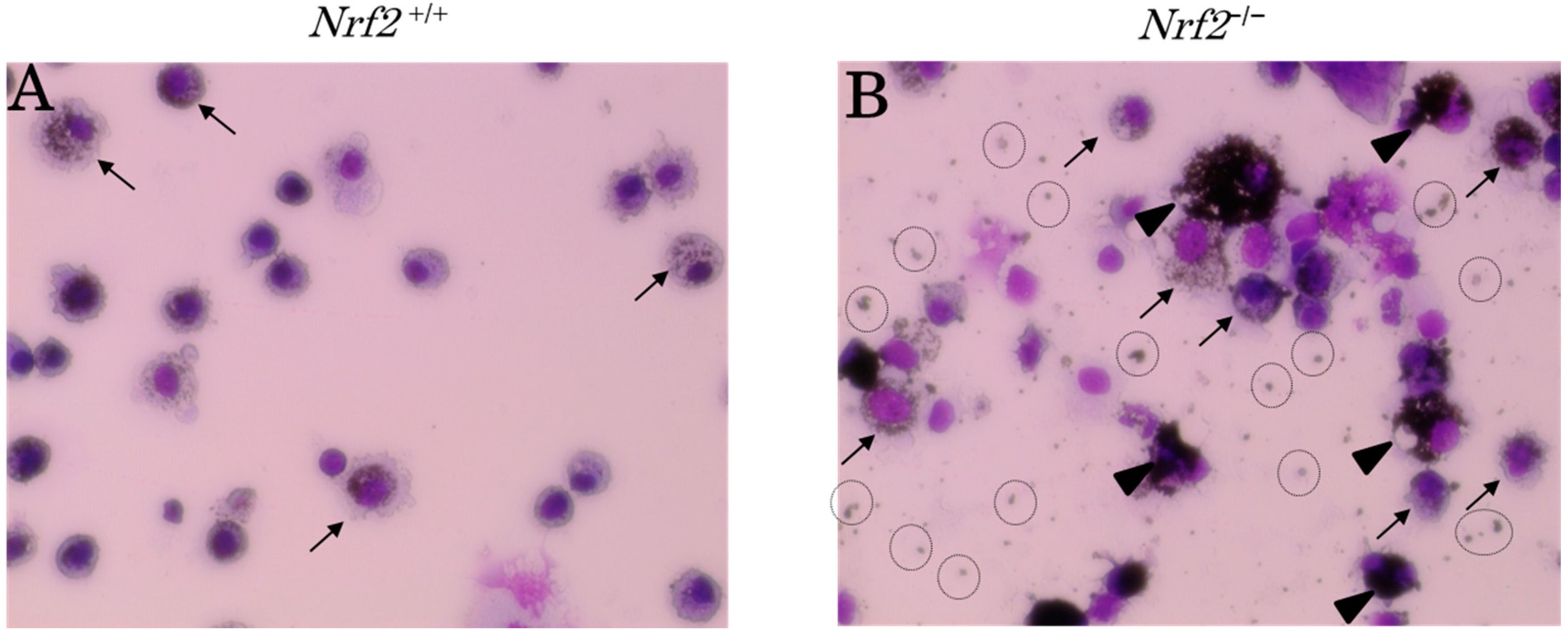
2.2. DEP-Laden Alveolar Macrophages
2.3. MIP-2, TNF-α, and TGF-β1 Concentrations in BALF
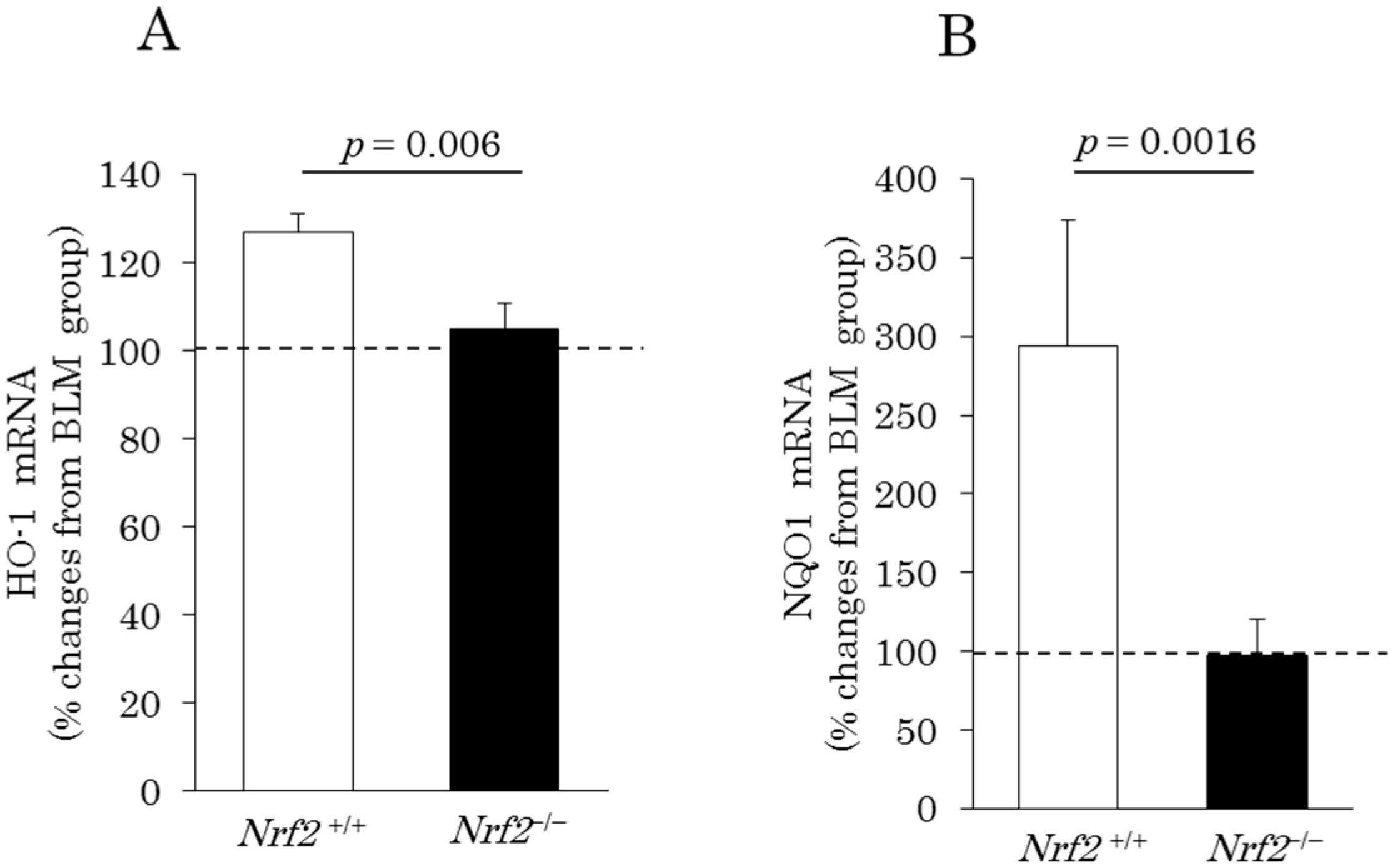
2.4. Induction of Pulmonary Antioxidant Enzyme mRNA Expression
2.5. Histopathologic Assessment
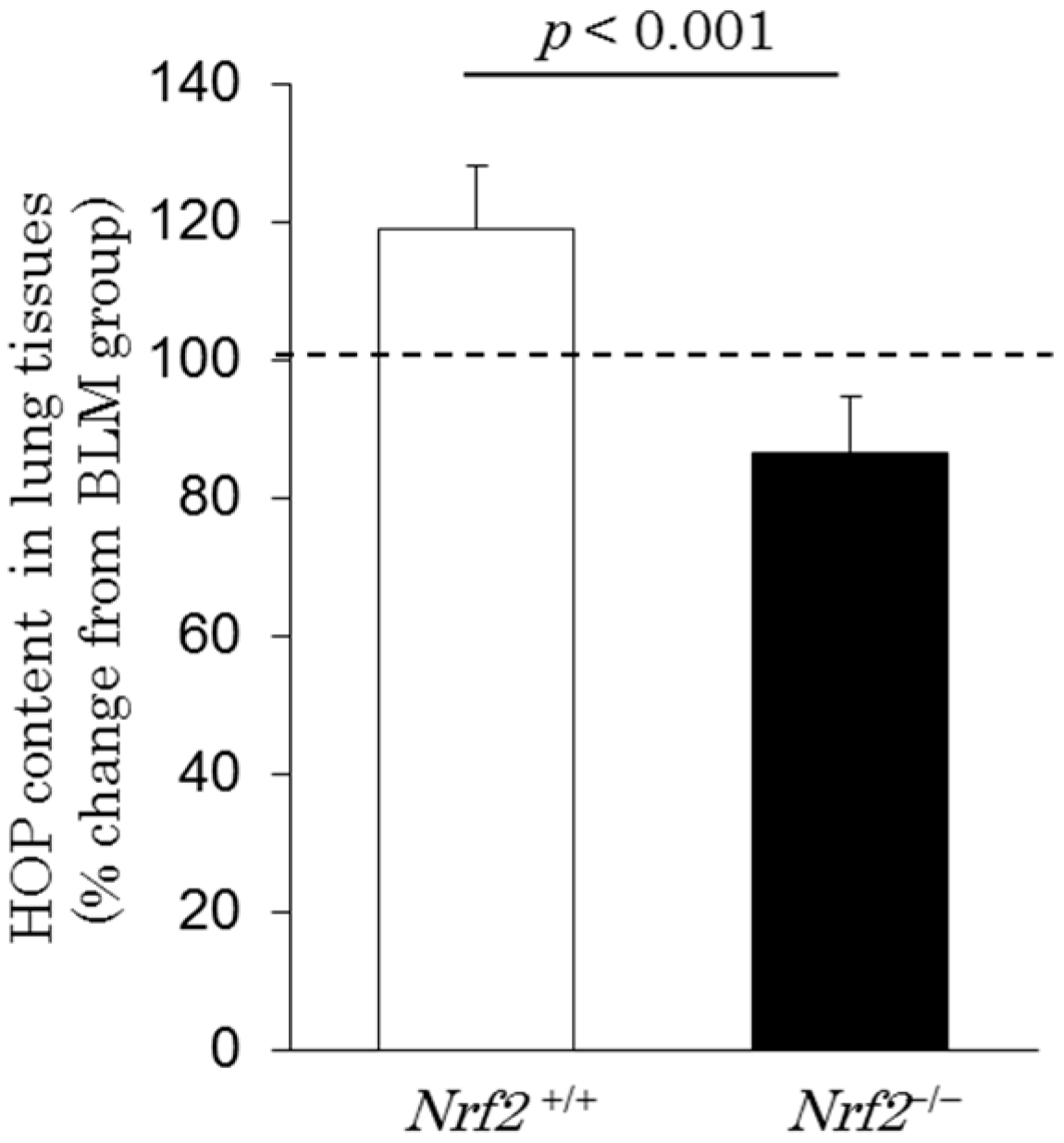
2.6. Hydroxyproline Content in Lung Tissues
3. Discussion
4 Materials and Methods
4.1. Animals
4.2. DE Exposure
4.3. Study Design
4.4. Histopathological Assay
4.5. Hydroxyproline Measurement
4.6. BAL and Cell Counts in BALF
4.7. Measurement of the BALF Cytokine Concentration
4.8. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Brunekreef, B.; Goldbohm, S.; Fischer, P.; van den Brandt, P.A. Association between mortality and indicators of traffic-related air pollution in the Netherlands: A cohort study. Lancet 2002, 360, 1203–1209. [Google Scholar] [CrossRef]
- Takizawa, H.; Ohtoshi, T.; Kawasaki, S.; Kohyama, T.; Desaki, M.; Kasama, T.; Kobayashi, K.; Nakahara, K.; Yamamoto, K.; Matsushima, K.; et al. Diesel exhaust particles induce NF-kappa B activation in human bronchial epithelial cells in vitro: Importance in cytokine transcription. J. Immunol. 1999, 162, 4705–4711. [Google Scholar] [PubMed]
- Takizawa, H.; Abe, S.; Okazaki, H.; Kohyama, T.; Sugawara, I.; Saito, Y.; Ohtoshi, T.; Kawasaki, S.; Desaki, M.; Nakahara, K.; et al. Diesel Exhaust Particles Upregulate Eotaxin Gene Expression in Human Bronchial Epithelial Cells via Nuclear Factor-Kappa B-Dependent Pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L1055–L1062. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Gon, Y.; Takeshita, I.; Matsumoto, K.; Jibiki, I.; Takizawa, H.; Kudoh, S.; Horie, T. Diesel Exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am. J. Respir. Crit. Care Med. 2000, 161, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J. Biol. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Venkatesan, M.I.; Miguel, A.; Kaplan, R.; Gujuluva, C.; Alam, J.; Nel, A. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J. Immunol. 2000, 165, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Alam, J.; Venkatesan, M.I.; Eiguren-Fernandez, A.; Schmitz, D.; Di Stefano, E.; Slaughter, N.; Killeen, E.; Wang, X.; Huang, A.; et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: Protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004, 173, 3467–3481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Kawada, T.; Matsumoto, A.; Azuma, A.; Kudoh, S.; Takizawa, H.; Sugawara, I. Airway inflammatory responses to oxidative stress induced by low-dose diesel exhaust particle exposure differ between mouse strains. Exp. Lung Res. 2007, 33, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Takizawa, H.; Azuma, A.; Kohyama, T.; Yamauchi, Y.; Kawada, T.; Kudoh, S.; Sugawara, I. The effects of oxidative stress induced by prolonged low-dose diesel exhaust particle exposure on the generation of allergic airway inflammation differ between BALB/c and C57BL/6 mice. Immunopharmacol. Immunotoxicol. 2009, 31, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Takizawa, H.; Azuma, A.; Kohyama, T.; Yamauchi, Y.; Takahashi, S.; Masayuki, Y.; Kawada, T.; Kudoh, S.; Sugawara, I. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin. Immunol. 2008, 128, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Helmers, R.A.; Galvin, J.R.; van Fossen, D.S.; Frees, K.L.; Dayton, C.S.; Burmeister, L.F.; Hunninghake, G.W. Determinants of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care 1994, 149, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.J.; Hunninghake, G.W. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001, 345, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; North, S.L.; Fells, G.A.; Hubbard, R.C.; Crystal, R.G. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J. Clin. Investig. 1987, 79, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Demedts, M.; Behr, J.; Buhl, R.; Costabel, U.; Dekhuijzen, R.; Jansen, H.M.; MacNee, W.; Thomeer, M.; Wallaert, B.; Laurent, F.; et al. IFIGENIA Study Group, High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2005, 353, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Fantone, J.C.; Phan, S.H. Oxygen metabolite detoxifying enzyme levels in bleomycin-induced fibrotic lungs. Free. Radic. Biol. Med. 1988, 4, 399–402. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kleeberger, S.R. Noblesse oblige: NRF2 functions in the airways. Am. J. Respir. Cell. Mol. Biol. 2014, 50, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Takizawa, H.; Azuma, A.; Kohyama, T.; Yamauchi, Y.; Takahashi, S.; Yamamoto, M.; Kawada, T.; Kudoh, S.; Sugawara, I. Nrf2 is closely related to allergic airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin. Immunol. 2010, 137, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Sun, J.; Li, W.; Yang, L.; Wen, L.; Wang, W.; Wang, X.; Collett, J.L., Jr.; Shi, Y.; et al. Severe haze episodes and seriously polluted fog water in Ji’nan. China Sci. Total Environ. 2014, 493, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Yoshikawa, T.; Ichinose, T.; Miyabara, Y.; Imaoka, K.; Sagai, M. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am. J. Respir. Crit. Care Med. 1997, 156, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Miyabara, Y.; Takano, H.; Ichinose, T.; Lim, H.B.; Sagai, M. Diesel exhaust enhances allergic airway inflammation and hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 1998, 157, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.B.; Hogaboam, C.M. Murine model of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L152–L160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Azuma, A.; Takahashi, S.; Usuki, J.; Kuniko, M.; Aoyama, A.; Kudoh, S. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration role in preventing lung injury and fibrosis in bleomycin-challenged mice. CHEST 2002, 122, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Reddy, S.P.; Yamamoto, M.; Kleeberger, S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004, 18, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Li, J.; Zhou, W.; Wu, S.; Xu, S.; Cui, K.; Zhang, D.D.; Liu, B. Artemisitene activates the Nrf2-dependent antioxidant response and protects against bleomycin-induced lung injury. FASEB J. 2016, 30, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Venosa, A.; Malaviya, R.; Gow, A.J.; Hall, L.; Laskin, J.D.; Laskin, D.L. Protective role of spleen-derived macrophages in lung inflammation, injury, and fibrosis induced by nitrogen mustard. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1487–L1498. [Google Scholar] [CrossRef] [PubMed]
- Withana, N.P.; Ma, X.; McGuire, H.M.; Verdoes, M.; van der Linden, W.A.; Ofori, L.O.; Zhang, R.; Li, H.; Sanman, L.E.; Wei, K.; et al. Non-invasive imaging of idiopathic pulmonary fibrosis using cathepsin protease probes. Sci. Rep. 2016, 6, 19755. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.; Kliment, C.R.; Metz, H.E.; Kim, K.H.; Kargl, J.; Agostini, B.A.; Crum, L.T.; Oczypok, E.A.; Oury, T.A.; Houghton, A.M. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J. Leukoc. Biol. 2015, 98, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Ma, J.K. The dual effect of the particulate and organic components of diesel exhaust particles on the alteration of pulmonary immune/inflammatory responses and metabolic enzymes. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2002, 20, 117–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Antonini, J.M.; Barger, M.W.; Butterworth, L.; Roberts, J.R.; Ma, J.K.H.; Castranova, V.; Ma, J.Y.C. Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of Listeria monocytogenes in rats. Environ. Health Perspect. 2001, 109, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Dong, C.C.; Ma, J.Y.; Roberts, J.R.; Antonini, J.M.; Ma, J.K. Suppression of phagocytic and bactericidal functions of rat alveolar macrophages by the organic component of diesel exhaust particles. J. Toxicol. Environ. Health A 2007, 70, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Blomberg, A.; Rudell, B.; Kelly, F.; Sandström, T.; Holgate, S.T.; Frew, A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999, 159, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, N.; Nordenhäll, C.; Salvi, S.S.; Mudway, I.; Söderberg, M.; Blomberg, A.; Helleday, R.; Levin, J.O.; Holgate, S.T.; Kelly, F.J.; et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur. Respir. J. 2004, 23, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Staitieh, B.S.; Egea, E.E.; Fan, X.; Azih, N.; Neveu, W.; Guidot, D.M. Activation of alveolar macrophages with interferon-γ promotes antioxidant defenses via the Nrf2-ARE pathway. J. Clin. Cell. Immunol. 2015, 6, 365. [Google Scholar] [PubMed]
- Lou, N.; Lennard Richard, M.L.; Yu, J.; Kindy, M.; Zhang, X.K. The Fli-1 transcription factor is a critical regulator for controlling the expression of chemokine C-X-C motif ligand 2 (CXCL2). Mol. Immunol. 2016, 81, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.P.; Belperio, J.A.; Moore, T.A.; Moore, B.B.; Arenberg, D.A.; Smith, R.E.; Burdick, M.D.; Kunkel, S.L.; Strieter, R.M. Neutralization of the CXC chemokine, macrophage inflammatory protein-2, attenuates bleomycin-induced pulmonary fibrosis. J. Immunol. 1999, 162, 5511–5518. [Google Scholar] [PubMed]
- Dworski, R.; Simon, H.U.; Hoskins, A.; Yousefi, S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J. Allergy Clin. Immunol. 2011, 127, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kinder, B.W.; Brown, K.K.; Schwarz, M.I.; Ix, J.H.; Kervitsky, A.; King, T.E., Jr. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008, 133, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Seriani, R.; de Souza, C.E.; Krempel, P.G.; Frias, D.P.; Matsuda, M.; Correia, A.T.; Ferreira, M.Z.; Alencar, A.M.; Negri, E.M.; Saldiva, P.H.; et al. Human bronchial epithelial cells exposed in vitro to diesel exhaust particles exhibit alterations in cell rheology and cytotoxicity associated with decrease in antioxidant defenses and imbalance in pro- and anti-apoptotic gene expression. Environ. Sci. Pollut. Res. Int. 2016, 23, 9862–9870. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Farver, C.F.; Vaszar, L.T.; Dempsey, O.J.; Popper, H.H.; Mani, H.; Capelozzi, V.L.; Fukuoka, J.; Kerr, K.M.; Zeren, E.H.; et al. Causes of pulmonary granulomas: A retrospective study of 500 cases from seven countries. J. Clin. Pathol. 2012, 65, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Yamamoto, M.; Sugawara, I. Significant reduction of granulomas in Nrf2-deficient mice infected with Mycobacterium tuberculosis. Indian J. Tuberc. 2010, 57, 108–113. [Google Scholar] [PubMed]
- Bonham, C.A.; Strek, M.E.; Patterson, K.C. From granuloma to fibrosis: Sarcoidosis associated pulmonary fibrosis. Curr. Opin. Pulm. Med. 2016, 22, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Yamashita, K.; Takemoto, N.; Ohshima, Y.; Tsukimoto, M.; Shinkai, Y.; Takeda, K.; Oshio, S.; Kojima, S. Diesel exhaust (DE) aggravates pathology of delayed-type hypersensitivity (DTH) induced by methyl-bovine serum albumin (mBSA) in mice. J. Toxicol. Sci. 2009, 34, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, M.; Sakata, C.; Tanaka, N.; Tabata, M.; Takeda, K.; Ihara, T.; Sugamata, M. Pathological study for the effects of in utero and postnatal exposure to diesel exhaust on a rat endometriosis model. J. Toxicol. Sci. 2011, 36, 493–498. [Google Scholar] [CrossRef] [PubMed]




| Chamber | CO (ppm) | SO2 (ppb) | NO (ppm) | NO2 (ppm) | NOx (ppm) | DEP (mg/m3) | DEP# /cc |
|---|---|---|---|---|---|---|---|
| Clean | 0.44 ± 0.17 | 0.64 ± 0.50 | 0.00 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 3 ± 1 |
| DE | 10.26 ± 2.72 | 21.03 ± 5.50 | 3.65 ± 0.84 | 1.91 ± 0.45 | 5.55 ± 1.26 | 1.02 ± 0.29 | 343,700 ± 2900 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-J.; Shimizu, T.; Shinkai, Y.; Hirata, Y.; Inagaki, H.; Takeda, K.; Azuma, A.; Yamamoto, M.; Kawada, T. Nrf2 Regulates the Risk of a Diesel Exhaust Inhalation-Induced Immune Response during Bleomycin Lung Injury and Fibrosis in Mice. Int. J. Mol. Sci. 2017, 18, 649. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030649
Li Y-J, Shimizu T, Shinkai Y, Hirata Y, Inagaki H, Takeda K, Azuma A, Yamamoto M, Kawada T. Nrf2 Regulates the Risk of a Diesel Exhaust Inhalation-Induced Immune Response during Bleomycin Lung Injury and Fibrosis in Mice. International Journal of Molecular Sciences. 2017; 18(3):649. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030649
Chicago/Turabian StyleLi, Ying-Ji, Takako Shimizu, Yusuke Shinkai, Yukiyo Hirata, Hirofumi Inagaki, Ken Takeda, Arata Azuma, Masayuki Yamamoto, and Tomoyuki Kawada. 2017. "Nrf2 Regulates the Risk of a Diesel Exhaust Inhalation-Induced Immune Response during Bleomycin Lung Injury and Fibrosis in Mice" International Journal of Molecular Sciences 18, no. 3: 649. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030649






