Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy
Abstract
:1. Introduction
2. Results and Discussion
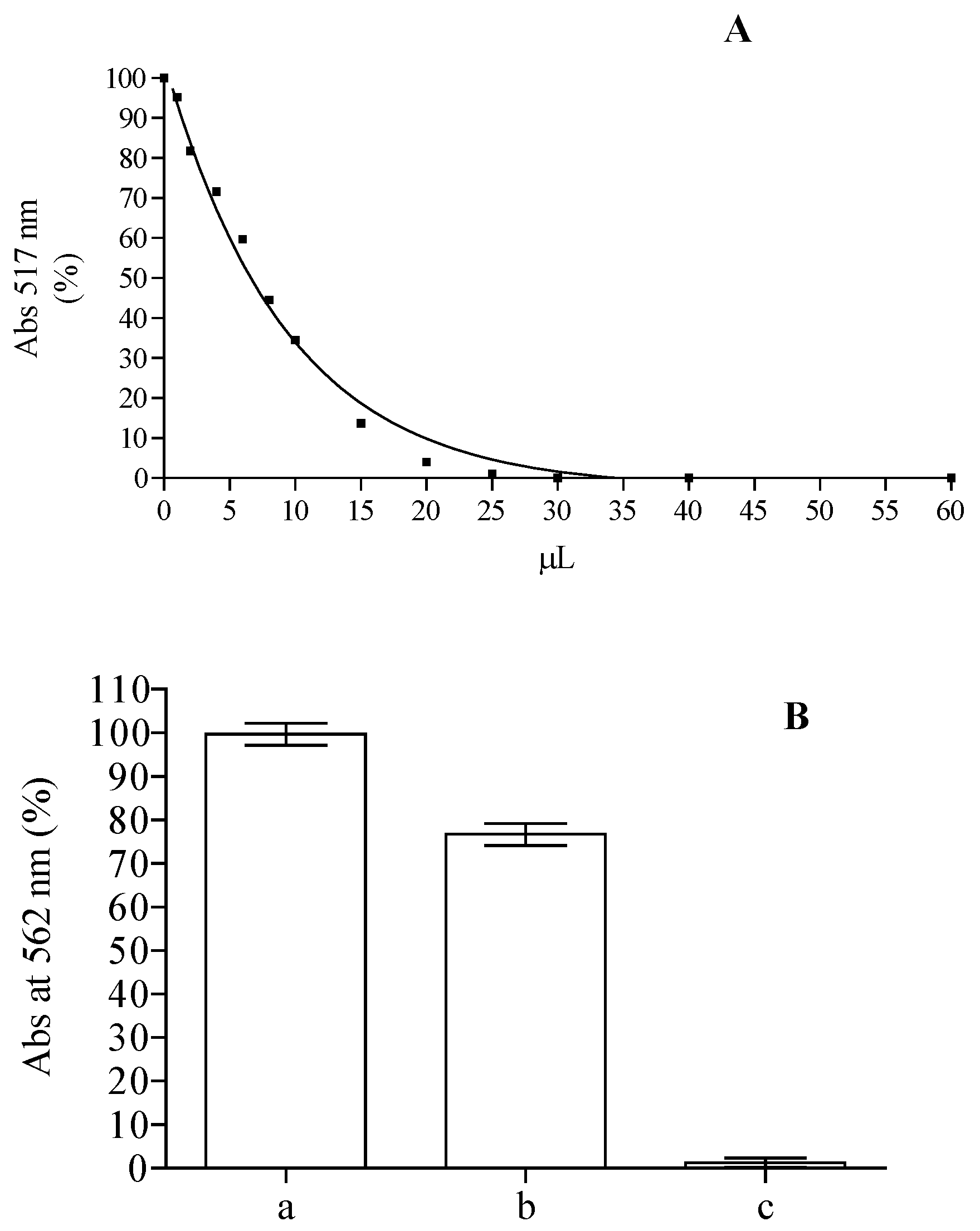
2.1. Phytochemical Screening and Antioxidant Activity
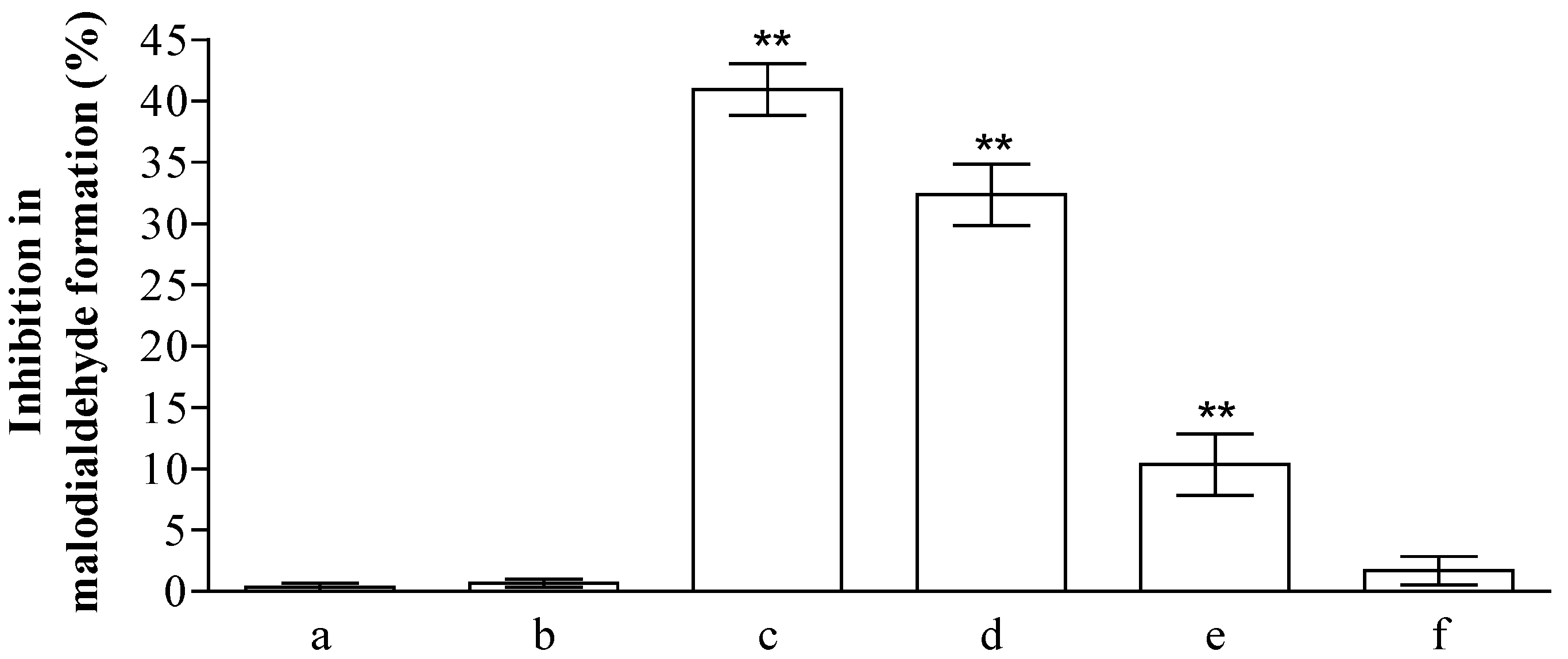
2.2. Analysis of Anti-Peroxidative and Cytoprotective Activity
2.3. RP-DAD-HPLC Separation and Identification of Flavonoids Derivatives
2.4. Antioxidant Capacity
3. Materials and Methods
3.1. Reagents, Chemicals, and Instrumentation
3.2. Chlorophyll and Carotenoid Pigments
3.3. Anthocyanins
3.4. Tartaric Acid Esters and Total Phenols
3.5. Reduced Glutathione
3.6. Ascorbic and Dehydroascorbic Acid
3.7. Enzyme Assays
3.8. Preparation of Methanol Extract
3.9. DPPH Radical Scavenging Assay
3.10. ABTS Radical Scavenging Assay
3.11. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.12. Ferrozine Assay
3.13. Flavonoids Profile Identification
3.14. Acid Hydrolysis
3.15. Erythrocytes Lipid Peroxidation Assay
3.16. Lymphocyte Isolation
3.17. Cytotoxicity Assays
3.18. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.19. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Divakara, B.N.; Upadhyaya, H.D.; Wani, S.P.; Gowda, C.L.L. Biology and genetic improvement of Jatropha curcas L.: A review. Appl. Energy 2010, 87, 732–742. [Google Scholar] [CrossRef]
- Francis, G.; Edinger, R.; Becker, K. A concept for simultaneous wasteland reclamation, fuel production, and socioeconomic development in degraded areas in India. Need, potential and perspectives of Jatropha plantations. Nat. Res. Forum 2005, 29, 12–24. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.N.; Fontenelea, A.V.; Ribeiro, R.V.; Viegasc, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K. Properties and use of Jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew. Energy 2003, 28, 239–248. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): A review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Ye, M.; Li, C.; Francis, G.; Makkar, H.P.S. Current situation and prospects of Jatropha curcas as a multipurpose tree in China. Agrofor. Syst. 2009, 76, 487–497. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, K.; Singh, J.S.; Kumar, A.; Singh, B.; Singh, R.P. Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renew. Sustain. Energy Rev. 2012, 16, 2870–2883. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Francis, G.; Becker, K. Preparation of protein concentrate from Jatropha curcas screw-pressed seed cake and toxic and antinutritional factors in protein concentrate. J. Sci. Food Agric. 2008, 88, 1542–1548. [Google Scholar] [CrossRef]
- Shetty, S.; Udupa, S.L.; Udupa, A.L.; Vollala, V.R. Woundhealing activities of bark extract of Jatropha curcas Linn. in albino rats. Saudi Med. J. 2006, 27, 1473–1476. [Google Scholar] [PubMed]
- Mujumdar, A.M.; Misar, A.V. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. J. Ethnopharmacol. 2004, 90, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, N.A.; Nyarko, A.K.; Addo, P.G.; Ofosuhene, M.; Dzokoto, C.; Marley, E.; Addae, M.M.; Ekuban, F.A. Evaluation of efficacy and safety of a herbal medicine used for the treatment of malaria. Phytother. Res. 2003, 17, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Fagbenro-Beyioku, A.F.; Oyibo, W.A.; Anuforom, B.C. Disinfectant/antiparasitic activities of Jatropha curcas. East Afr. Med. J. 1998, 75, 508–511. [Google Scholar] [PubMed]
- Kalimuthu, K.; Vijayakumar, S.; Senthilkumar, R. Antimicrobial activity of the biodesel plant, Jatropha curcas. Intern. J. Pharm. Biol. Sci. 2010, 1, 1–5. [Google Scholar]
- Adebowale, K.O.; Adedire, C.O. Chemical composition and insecticidal properties of the underutilized Jatropha curcas seed oil. Afr. J. Biotechnol. 2006, 5, 901–906. [Google Scholar]
- Igbinosa, O.O.; Igbinosa, I.H.; Chigor, V.N.; Uzunuigbe, O.E.; Oyedemi, S.O.; Odjadiare, E.E.; Okoh, A.I.; Igbinosa, E.O. Polyphenolic contents and antioxidant potential of stem bark extracts of Jatropha curcas (Linn). Int. J. Mol. Sci. 2011, 12, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Muangman, S.; Thippornwong, M.; Tohtong, R. Anti-metastatic effects of curcusone B, a diterpene from Jatropha curcas. In Vivo 2005, 19, 265–268. [Google Scholar] [PubMed]
- Sabandar, C.W.; Ahmat, N.; Mahf Jaafar, F.; Sahidin, I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Asase, A.; Oteng-Yeboah, A.A.; Odamtten, G.T.; Simmonds, M.S.J. Ethnobotanical study of some Ghananian anti-malarial plants. J. Ethnopharmacol. 2005, 99, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Sah, N.K.; Sharma, P.B. Therapeutic biology of Jatropha curcas: A mini review. Curr. Pharm. Biotechnol. 2008, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Aly, H.F.; Abd-Alla, H.; Saad, S.A. Bioactive flavonoid glycosides and antidiabetic activity of Jatropha curcas on streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 143–156. [Google Scholar]
- Knnappan, N.; Jaikumar, S.; Manavalan, R.; Muthu, A.K. Antiulcer activity of methanolic extract of Jatropha curcas (Linn.) on aspirin-induced gastric lesions in wistar rats. Pharmacologyonline 2008, 1, 279–293. [Google Scholar]
- Dahake, R.; Roy, S.; Patil, D.; Rajopadhye, S.; Chowdhary, A.; Deshmukh, R.A. Potential anti-HIV activity of Jatropha curcas Linn. leaf extracts. J. Antivir. Antretrovir. 2013, 5, 160–165. [Google Scholar] [CrossRef]
- Patil, D.Y.; Roy, S.; Dahake, R.; Rajopadhye, S.; Kothari, S.; Deshmukh, R.; Chowdhary, A. Evaluation of Jatropha curcas Linn. leaf extracts for its cytotoxicity and potential to inhibit hemagglutinin protein of influenza virus. Indian J. Virol. 2013, 24, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Settineri, G.; Panuccio, M.R.; Muscolo, A. Jatropha curcas sludge valorization. Procedia Soc. Behav. Sci. 2016, 223, 865–887. [Google Scholar] [CrossRef]
- De Rossi, A.; Vescio, R.; Russo, D.; Macrì, G. Potential use of Jatropha curcas L. on marginal lands of southern Italy. Procedia Soc. Behav. Sci. 2016, 223, 770–775. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Photosynthetic acclimation to high temperatures associated with heat tolerance in creeping bentgrass. J. Plant Physiol. 2008, 165, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell. Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.A.; Sandalio, L.M.; Corpas, F.J.; Barroso, J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging and role in cell signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Panneerselvam, R. Alterations in non-enzymatic antioxidant components of Catharanthus roseus exposed to paclobutrazol, gibberellic acid and Pseudomonas fluorescens. Plant Omics J. 2009, 2, 30–40. [Google Scholar]
- Gechev, T.S.; van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Das, M.K.; Roychoudhury, A. ROS and responses of antioxidant as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, M.R.; Fazio, A.; Papalia, T.; Barreca, D. Antioxidant properties and flavonoid profile in leaves of Calabrian Lavandula multifida L., an autochthon plant of Mediterranean Southern regions. Chem. Biodivers. 2016, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistachia vera L. variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Laganà, G.; Leuzzi, U.; Bellocco, E. C- and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: Identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem. 2016, 196, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3-O-galactoside in ripe pistachio (Pistachia vera L. variety Bronte) hulls: Identification and evaluation of its antioxidant and cytoprotective activities. J. Funct. Foods 2016, 27, 376–385. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Toscano, G.; Calandra, P.; Kiselev, M.A.; Lombardo, D.; Bellocco, E. The interaction and binding of flavonoids to human serum albumin modify its conformation, stability and resistance against aggregation and oxidative injuries. BBA Gen. Subj. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Laganà, G.; Tellone, E.; Ficarra, S.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Influences of flavonoids on erythrocyte membrane and metabolic implication through anionic exchange modulation. J. Membr. Biol. 2009, 230, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Annona cherimola Mill. (Annonaceae). Food Res. Int. 2011, 44, 2302–2310. [Google Scholar] [CrossRef]
- Bellocco, E.; Barreca, D.; Laganà, G.; Leuzzi, U.; Tellone, E.; Ficarra, S.; Kotyk, A.; Galtieri, A. Influence of l-rhamnosyl-d-glucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–966. [Google Scholar] [CrossRef]
- Tomar, N.S.; Sharma, M.; Agarwal, R.M. Phytochemical analysis of Jatropha curcas L. during different seasons and developmental stages and seedling growth of wheat (Triticum aestivum L) as affected by extracts/leachates of Jatropha curcas L. Physiol. Mol. Biol. Plants 2015, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Oskoueian, E.; Abdullah, N.; Saad, W.Z.; Omar, A.R.; Ahmad, S.; Kuan, W.B.; Zolkifli, N.A.; Hendra, R.; Ho, Y.W. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J. Med. Plants Res. 2011, 5, 49–57. [Google Scholar]
- Najda, A.; Almehemdi, A.F.; Zabar, A.F. Chemical composition and nutritional value of Jatropha curcas L. leaves. J. Genet. Environ. Res. Conserv. 2013, 1, 221–226. [Google Scholar]
- Diwani, G.E.; Rafie, S.E.; Hawash, S. Antioxidant activity of extracts obtained from residues of nodes leaves stem and root of Egyptian Jatropha curcas. Afr. J. Pharm. Pharmacol. 2009, 3, 521–530. [Google Scholar]
- Rampadarath, S.; Puchooa, D.; Ranghoo-Sanmukhiya, V.M. A comparison of polyphenolic content, antioxidant activity and insecticidal properties of Jatropha species and wild Ricinus communis L. found in Mauritius. Asian Pac. J. Trop. Med. 2014, 7, S384–S390. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Moharram, F.A.; Gaara, A.H.; El-Safty, M.M. Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Z. Naturforsch. 2009, 64, 495–501. [Google Scholar] [CrossRef]
- Masaoud, I.M.; Ripperger, H.; Porzel, A.; Adam, G. Flavonol glycosides from Jatropha variegate. Z. Prakt. Chem. 1995, 337, 43–45. [Google Scholar] [CrossRef]
- Subramanian, S.S.; Nagarajan, S.; Sulochana, N. Flavonoids of the leaves of Jatropha gossypiifolia. Phytochemistry 1971, 10, 1690. [Google Scholar] [CrossRef]
- Debnath, M.; Bisen, P.S. Jatropha curcas L., a multipurpose stress resistant plant with a potential for ethnomedicine and renewable energy. Curr. Pharm. Biotechnol. 2008, 9, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Guo, Y.; Fu, R.; Peng, T.; Zhang, Y.; Chen, F. Antioxidant activity of flavonoids from leaves of Jatropha curcas. Sci. Asia 2014, 40, 193–197. [Google Scholar] [CrossRef]
- Félix-Silva, J.; Souza, T.; Menezes, Y.A.S.; Cabral, B.; Câmara, R.B.G.; Silva-Junior, A.A.; Rocha, H.A.O.; Rebecchi, I.M.M.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Aqueous leaf extract of Jatropha gossypiifolia L. (Euphorbiaceae) inhibits enzymatic and biological actions of Bothrops jararaca snake venom. PLoS ONE 2014, 9, e104952. [Google Scholar] [CrossRef] [PubMed]
- Pilon, A.C.; Carneiro, R.L.; Carnevale Neto, F.; Bolzani, V.S.; Castro-Gamboa, I. Interval multivariate curve resolution in the dereplication of HPLC-DAD data from Jatropha gossypifolia. Phytochem. Anal. 2013, 24, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Rofida, S. Antioxidant activity of Jatropha curcas and Jatropha gossypifolia by DPPH method. Farmasains 2015, 2, 281–284. [Google Scholar]
- Fu, R.; Zhang, Y.; Guo, Y.; Liu, F.; Chen, F. Determination of phenolic contents and antioxidant activities of extracts of Jatropha curcas L. seed shell, a by-product, a new source ofnatural antioxidant. Ind. Crops Prod. 2014, 58, 265–270. [Google Scholar] [CrossRef]
- Romani, A.; Mancini, P.; Tatti, S.; Vincieri, F. Polyphenols and polysaccharidies in Tuscan grapes and wines. Ital. J. Food Sci. 1996, 8, 13–24. [Google Scholar]
- Singleton, V.R.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, F.; Jouve, H.M.; Gagnon, J.; Gaillard, J.; Pelmont, J. Purification and properties of a catalase from potato tubers (Solanum tuberosum). Plant Sci. 1990, 72, 19–26. [Google Scholar] [CrossRef]
- Panda, S.K.; Singha, L.B.; Khan, M.H. Does aluminium phytotoxicity induce oxidative stress in greengram (Vigna radiata). Bulg. J. Plant Physiol. 2003, 29, 77–86. [Google Scholar]
- Doulis, A.G.; Debian, N.; Kingston-Smith, A.H.; Foyer, C.H. Differential localization of antioxidants in maize leaves. Plant Physiol. 1997, 114, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Amako, K.; Chen, G.X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar]
- Molineux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–23. [Google Scholar] [PubMed]
- Dorman, H.J.D.; Kosar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Hollman, P.C.H.; Venema, D.P. Optimization of quantitative HPLC determination of potentially anticarcinogenic flavonoids in fruits and vegetables. J. Agric. Food Chem. 1992, 40, 1591–1598. [Google Scholar] [CrossRef]
- Yagi, K.; Rastogi, R. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annu. Rev. Biochem. 1979, 95, 351–358. [Google Scholar]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]


| Texture | pH | E.C. (mS/cm) | Total Carbonates (%) | TOC (%) | SOM (%) | N (g/kg) | C/N |
|---|---|---|---|---|---|---|---|
| Loam-sandy | 8.20 | 1.65 | 2.00 | 14.06 | 2.60 | 1.82 | 7.73 |
| Phytochemical Screening of Jatropha curcas L. Leaf | Value |
|---|---|
| Chlorophyll a (mg·g−1 Fresh Weight) | 1.60 ± 0.10 |
| Chlorophyll b (mg·g−1 Fresh Weight) | 0.90 ± 0.03 |
| Catalase (CAT) activity (nmol H2O2·g−1 Fresh Weight) | 14.75 ± 1.20 |
| Peroxidases (POX) activity (µmol guaiacol·g−1 Fresh Weight) | 1.06 ± 0.04 |
| Ascorbate peroxidase (APX) activity (µmol H2O2·g−1 Fresh Weight) | 1.30 ± 0.04 |
| Dehydroascorbate reductase (DHA-Rd) activity (µmol ASA·g−1 Fresh Weight) | 0.77 ± 7.10 |
| Ascorbic acid (ASA) (µmol ascorbic acid/g Dry Weight) | 3.78 ± 0.19 |
| Dehydroascorbic acid (µmol dehydroascorbic acid/g Dry Weight) | 2.34 ± 0.20 |
| Reduced glutathione (µmol GSH/g Dry Weight) | 1.75 ± 0.14 |
| Total phenols (mg tannic acid/g Dry Weight) | 7.36 ± 0.60 |
| Total carotenoids (mg/g Fresh Weight) | 0.20 ± 0.03 |
| Anthocyanins (µg anthocyanin·g−1 Fresh Weight) | 9.42 ± 2.30 |
| Tartaric acid esters derivatives (µg caffeic acid·g−1 Fresh Weight) | 23.00 ± 0.10 |
| Compounds | mg/kg F.W. |
|---|---|
| Vicenin-2 | 3.7 ± 0.41 |
| Stellarin-2 | 1.2 ± 0.23 |
| Vitexin | 6.0 ± 0.52 |
| Isovitexin | 0.13 ± 0.04 |
| Isorhoifolin | Trace |
| Rhoifolin | 2.2 ± 0.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papalia, T.; Barreca, D.; Panuccio, M.R. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030660
Papalia T, Barreca D, Panuccio MR. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. International Journal of Molecular Sciences. 2017; 18(3):660. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030660
Chicago/Turabian StylePapalia, Teresa, Davide Barreca, and Maria Rosaria Panuccio. 2017. "Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy" International Journal of Molecular Sciences 18, no. 3: 660. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18030660







