Melatonin Reduces Angiogenesis in Serous Papillary Ovarian Carcinoma of Ethanol-Preferring Rats
Abstract
:1. Introduction
2. Results
2.1. Anatomopathological Analysis of OC (Ovarian Cancer) and Melatonin Levels
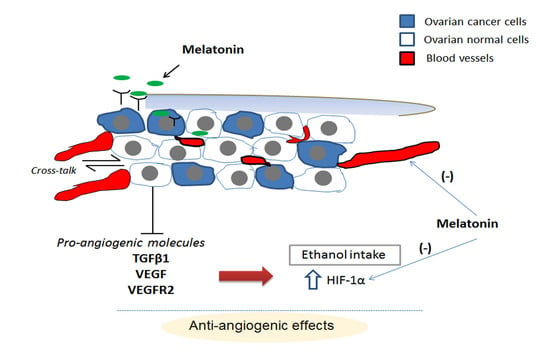
2.2. Melatonin Therapy Reduced the Vessel Density in OC
2.3. MT1 (Type 1 Melatonin Receptor) Is Upregulated by Melatonin in OC While Transforming Growth Factor (TGF)-β1 Levels Are Downregulated
2.4. Melatonin Downregulates VEGF (Vascular Endothelial Growth Factor) and VEGFR2 (VEGF Receptor 2), Even in the Presence of Ethanol
2.5. Hypoxia-Inducible Factor (HIF)-1α Is Downregulated by Melatonin Only in the Presence of EtOH Intake
2.6. Melatonin and Ethanol Differentially Modulated the VEGF/VEGFR/HIF-1α Pathway
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Intraovarian Injection of DMBA for Tumor Initiation
4.4. Melatonin Levels
4.5. Histopathology
4.6. Vascular Density
4.7. Immunofluorescence
4.8. Immunohistochemistry
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. Pathogenesis of ovarian cancer. Lessons from morphology and molecular biology and their clinical implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160. [Google Scholar] [PubMed]
- Chuffa, L.G.; Fioruci-Fontanelli, B.A.; Mendes, L.O.; Ferreira Seiva, F.R.; Martinez, M.; Fávaro, W.J.; Domeniconi, R.F.; Pinheiro, P.F.; Delazari Dos Santos, L.; Martinez, F.E. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 2015, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Carrara, O.; Oger-Jeannin, V.; Desechalliers, J.P. Disorders of the hypothalamo-hypophyseal-ovarian axis in chronic alcoholic women. Rev. Med. Int. 1993, 14, 9–13. [Google Scholar] [CrossRef]
- Henderson, J.; Gray, R.; Brocklehurst, P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG 2007, 114, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Berstad, P.; Ma, H.; Bernstein, L.; Ursin, G. Alcohol intake and breast cancer risk among young women. Breast Cancer Res. Treat. 2008, 108, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Fioruci-Fontanelli, B.A.; Mendes, L.O.; Fávaro, W.J.; Pinheiro, P.F.; Martinez, M.; Martinez, F.E. Characterization of chemically induced ovarian carcinomas in an ethanol-preferring rat model: Influence of long-term melatonin treatment. PLoS ONE 2013, 8, e81676. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Lupi Júnior, L.A.; Seiva, F.R.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.; Dos Santos, L.D.; Martinez, F.E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.J.; Al-Attar, A.; Rolland, P.; Scott, I.V.; Deen, S.; Liu, D.T.; Spendlove, I.; Durrant, L.G. Vascular endothelial growth factor expression in ovarian cancer: A model for targeted use of novel therapies? Clin. Cancer Res. 2008, 14, 3030–3035. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Teruya-Feldstein, J.; Wu, Y.; Zhu, Z.; Hicklin, D.J.; Moore, M.A. Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood 2004, 104, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Germain, S.; Monnot, C.; Muller, L.; Eichmann, A. Hypoxia-driven angiogenesis: Role of tip cells and extracellular matrix scaffolding. Curr. Opin. Hematol. 2010, 17, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Activation of the HIF pathway in cancer. Curr. Opin. Genet. 2001, 11, 293–299. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Reinthaller, A. Antiangiogenic therapies in ovarian cancer. Memo 2016, 9, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Liu, J.J.; Wang, C.Z.; Lin, B.; Hao, Y.Y.; Wang, Y.F.; Gao, S.; Qi, Y.; Zhang, S.L.; Iwamori, M. Expression and correlation of Lewis y antigen and TGF-B1 in ovarian epithelial carcinoma. Oncol. Rep. 2012, 27, 1065–1071. [Google Scholar] [PubMed]
- Do, T.V.; Kubba, L.A.; Du, H.; Sturgis, C.D.; Woodruff, T.K. Transforming growth factor-β1, transforming growth factor-β2, and transforming growth factor-β3 enhance ovarian cancer metastatic potential by inducing a Smad3-dependent epithelial-to-mesenchymal transition. Mol. Cancer Res. 2008, 6, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Stehle, J.H.; Saade, A.; Rawashdeh, O.; Ackermann, K.; Jilg, A.; Sebestény, T.; Maronde, E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res. 2011, 51, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Mechanisms of cancer inhibition by melatonin. J. Pineal Res. 2004, 3, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Ordoñez, R.; Benet, M.; Jover, R.; García-Palomo, A.; Mauriz, J.L.; González-Gallego, J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br. J. Cancer. 2013, 109, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.; Ke, C.; Yin, M.; Li, Z.; Fan, L.; Zhang, W.; Zhang, H.; Zhao, F.; Zhou, X.; et al. Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. J. Proteome Res. 2013, 12, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jang, W.J.; Yi, E.Y.; Jang, J.Y.; Jung, Y.; Jeong, J.W.; Kim, Y.J. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1α stabilization under hypoxia. J. Pineal Res. 2010, 48, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Arbab, A.S.; Ferreira, L.C.; Borin, T.F.; Varma, N.R.; Iskander, A.S.; Shankar, A.; Ali, M.M.; de Campos Zuccari, D.A. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE 2014, 9, e85311. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A. Antiangiogenic agents should be integrated into the standard treatment for patients with ovarian cancer. Ann. Oncol. 2011, 22, viii65–viii68. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Deng, L.; Li, J.; Zhang, Y.; Hu, L. The prognostic value of vascular endothelial growth factor in ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2013, 128, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.M.; Martinez, M.; Camargo, I.C.; Domeniconi, R.F.; Martinez, F.E.; Chuffa, L.G. Melatonin attenuates Her-2, p38 MAPK, p-AKT, and mTOR levels in ovarian carcinoma of ethanol-preferring rats. J. Cancer 2014, 5, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, S.J.; Kim, B.; Yun, S.M.; Choi, D.Y.; Kim, S.H. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J. Pineal Res. 2012, 52, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Alves, M.S.; Martinez, M.; Camargo, I.C.; Pinheiro, P.F.; Domeniconi, R.F.; Júnior, L.A.; Martinez, F.E. Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr. Relat. Cancer 2016, 23, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Mascia, F.; Gualano, L.; di Bella, L. Melatonin anticancer effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef] [PubMed]
- Orendáš, P.; Kubatka, P.; Bojková, B.; Kassayová, M.; Kajo, K.; Výbohová, D.; Kružliak, P.; Péč, M.; Adamkov, M.; Kapinová, A.; et al. Melatonin potentiates the anti-tumour effect of pravastatin in rat mammary gland carcinoma model. Int. J. Exp. Pathol. 2014, 95, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, K.; Pula, B.; Zemla, A.; Kobierzycki, C.; Kedzia, W.; Nowak-Markwitz, E.; Spaczynski, M.; Zabel, M.; Podhorska-Okolow, M.; Dziegiel, P. Expression of the MT1 melatonin receptor in ovarian cancer cells. Int. J. Mol. Sci. 2014, 15, 23074–23089. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G.; Melan, M.A.; Latimer, J.J.; Witt-Enderby, P.A. Melatonin and breast cancer: Cellular mechanisms, clinical studies and future perspectives. Expert Rev. Mol. Med. 2009, 11, e5. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Haldar, C.; Ortmann, O. Antiestrogens modulate MT1 melatonin receptor expression in breast and ovarian cancer cell lines. Oncol. Rep. 2006, 15, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Lee, M.T.; Ke, F.C.; Lee, P.P.; Huang, C.J.; Ip, M.M.; Chen, L.; Hwang, J.J. TGFβ1 stimulates the secretion of matrix metalloproteinase 2 (MMP2) and the invasive behavior in human ovarian cancer cells, which is suppressed by MMP inhibitor BB3103. Clin. Exp. Metastasis 2000, 18, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Bellone, S.; Ravaggi, A.; Roman, J.; Smith, C.V.; Pecorelli, S.; Cannon, M.J.; Parham, G.P. Increased levels of interleukin-10 and transforming growth factor-β in the plasma and ascitic fluid of patients with advanced ovarian cancer. BJOG 2001, 108, 804–808. [Google Scholar] [PubMed]
- Proietti, S.; Cucina, A.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Lisi, E.; Bizzarri, M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J. Pineal Res. 2011, 50, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Farzan, T.; Harrington, S.C.; Krempski, J.W.; Weroha, S.J.; Hou, X.; Kalli, K.R.; Wong, T.W.; Haluska, P. Dual HER/VEGF receptor targeting inhibits in vivo ovarian cancer tumor growth. Mol. Cancer Ther. 2013, 12, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, J.; Zhao, X. Expression of VEGF-D in epithelial ovarian cancer and its relationship to lymphatic metastasis. Asia Pac. J. Clin. Oncol. 2016, 12, e161–e166. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Bie, Z.; Zhang, S.; Li, A. Paclitaxel targets VEGF-mediated angiogenesis in ovarian cancer treatment. Am. J. Cancer Res. 2016, 6, 1624–1635. [Google Scholar] [PubMed]
- Dai, M.; Cui, P.; Yu, M.; Han, J.; Li, H.; Xiu, R. Melatonin modulates the expression of VEGF and HIF-1α induced by CoCl2 in cultured cancer cells. J. Pineal Res. 2008, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, M.S.; Suh, S.I.; Baek, W.K. Melatonin downregulates HIF-1α expression through inhibition of protein translation in prostate cancer cells. J. Pineal Res. 2009, 46, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, V.; Gonzalez, A.; Alonso-Gonzalez, C.; Martinez-Campa, C.; Cos, S. Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J. Pineal Res. 2013, 54, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.R.; Tomada, I.M.; Assunção, M.M.; Marques, F.A.; Almeida, H.M.; Andrade, J.P. Effects of chronic red wine consumption on the expression of vascular endothelial growth factor, angiopoietin 1, angiopoietin 2, and its receptors in rat erectile tissue. J. Food Sci. 2010, 75, H79–H86. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Komai, K.; Nishida, T.; Kamura, T.; Kojiro, M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer 2004, 101, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Spannuth, W.A.; Nick, A.M.; Jennings, N.B.; Armaiz-Pena, G.N.; Mangala, L.S.; Danes, C.G.; Lin, Y.G.; Merritt, W.M.; Thaker, P.H.; Kamat, A.A.; et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int. J. Cancer. 2009, 124, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Heukamp, L.C.; Siobal, M.; Schottle, J.; Wieczorek, C.; Peifer, M.; Frasca, D.; Koker, M.; Konig, K.; Meder, L.; et al. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J. Clin. Investig. 2013, 123, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Lourenço, M.R.; Doho, G.M.; Grígolo, I.H.; Gelaleti, G.B.; Ferreira, L.C.; Borin, T.F.; Moschetta, M.G.; Pires de Campos Zuccari, D.A. Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anticancer Agents Med. Chem. 2016, 16, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Milkiewicz, M.; Ispanovic, E.; Doyle, J.L.; Haas, T.L. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int. J. Biochem. Cell Biol. 2006, 38, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing system: A review. Biochim. Biophys. Acta 2016, 1865, 176–183. [Google Scholar] [PubMed]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62, e12370. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Seiva, F.R.; Fávaro, W.J.; Teixeira, G.R.; Amorim, J.P.; Mendes, L.O.; Fioruci, B.A.; Pinheiro, P.F.; Fernandes, A.A.; Franci, J.A.; et al. Melatonin reduces LH, 17 β-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 2011, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Seiva, F.R.; Fávaro, W.J.; Amorim, J.P.; Teixeira, G.R.; Mendes, L.O.; Fioruci-Fontanelli, B.A.; Pinheiro, P.F.; Martinez, M.; Martinez, F.E. Melatonin and ethanol intake exert opposite effects on circulating estradiol and progesterone and differentially regulate sex steroid receptors in the ovaries, oviducts, and uteri of adult rats. Reprod. Toxicol. 2013, 39, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Amorim, J.P.; Teixeira, G.R.; Mendes, L.O.; Fioruci, B.A.; Pinheiro, P.F.; Seiva, F.R.; Novelli, E.L.; de Mello Júnior, W.; Martinez, M.; et al. Long-term exogenous melatonin treatment modulates overall feed efficiency and protects ovarian tissue against injuries caused by ethanol-induced oxidative stress in adult UChB rats. Alcohol. Clin. Exp. Res. 2011, 35, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Amorim, J.P.; Teixeira, G.R.; Mendes, L.O.; Fioruci, B.A.; Pinheiro, P.F.; Seiva, F.R.; Novelli, E.L.; Mello Júnior, W.; Martinez, M.; et al. Long-term melatonin treatment reduces ovarian mass and enhances tissue antioxidant defenses during ovulation in the rat. Braz. J. Med. Biol. Res. 2011, 44, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, P.B.; Davis, J.R.; Bedrnicek, J.B.; Marion, S.L.; Christian, P.J.; Barton, J.K.; Brewer, M.A. Ovarian neoplasm development by 7,12-dimethylbenz[a]anthracene (DMBA) in a chemically-induced rat model of ovarian failure. Gynecol. Oncol. 2009, 112, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]







| Target Proteins | Treatments | |||
|---|---|---|---|---|
| OC | OC + Mel | OC + EtOH | OC + EtOH + Mel | |
| MT1 | + | ++ | + | ++ |
| TGFβ1 | +++ | + | +++ | ++ |
| VEGF | ++ | + | +++ | + |
| VEGFR1 | +/++ | +/++ | + | 0/+ |
| VEGFR2 | +++ | + | +++ | 0/+ |
| HIF-1α | ++ | + | ++/+++ | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zonta, Y.R.; Martinez, M.; Camargo, I.C.C.; Domeniconi, R.F.; Lupi Júnior, L.A.; Pinheiro, P.F.F.; Reiter, R.J.; Martinez, F.E.; Chuffa, L.G.A. Melatonin Reduces Angiogenesis in Serous Papillary Ovarian Carcinoma of Ethanol-Preferring Rats. Int. J. Mol. Sci. 2017, 18, 763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040763
Zonta YR, Martinez M, Camargo ICC, Domeniconi RF, Lupi Júnior LA, Pinheiro PFF, Reiter RJ, Martinez FE, Chuffa LGA. Melatonin Reduces Angiogenesis in Serous Papillary Ovarian Carcinoma of Ethanol-Preferring Rats. International Journal of Molecular Sciences. 2017; 18(4):763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040763
Chicago/Turabian StyleZonta, Yohan Ricci, Marcelo Martinez, Isabel Cristina C. Camargo, Raquel F. Domeniconi, Luiz Antonio Lupi Júnior, Patricia Fernanda F. Pinheiro, Russel J. Reiter, Francisco Eduardo Martinez, and Luiz Gustavo A. Chuffa. 2017. "Melatonin Reduces Angiogenesis in Serous Papillary Ovarian Carcinoma of Ethanol-Preferring Rats" International Journal of Molecular Sciences 18, no. 4: 763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040763