Detection of (1,3)-β-d-Glucan for the Diagnosis of Invasive Fungal Infection in Liver Transplant Recipients
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Prophylaxis Treatment
2.3. Invasive Fungal Infections
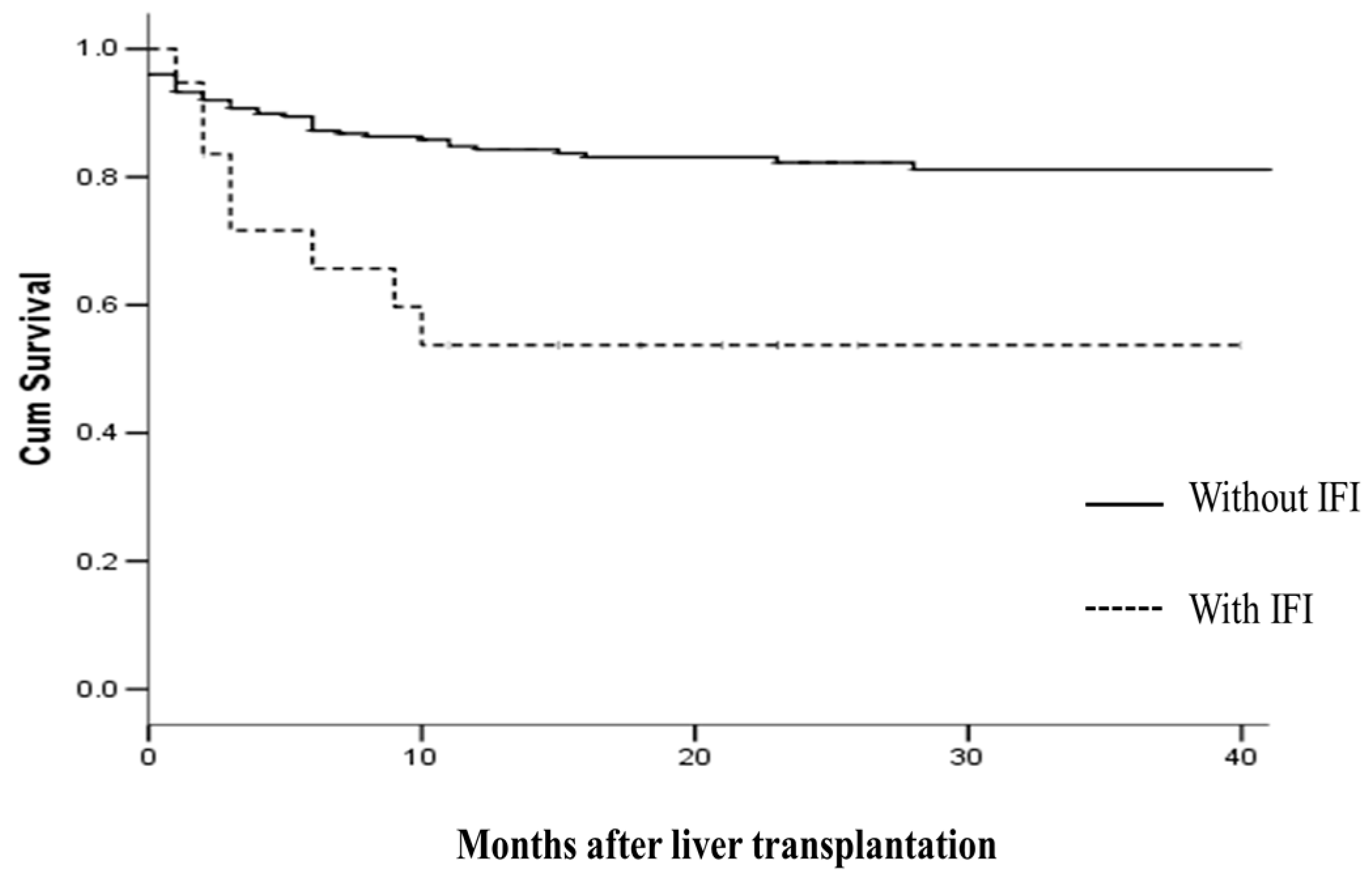
2.4. IFI and Survival
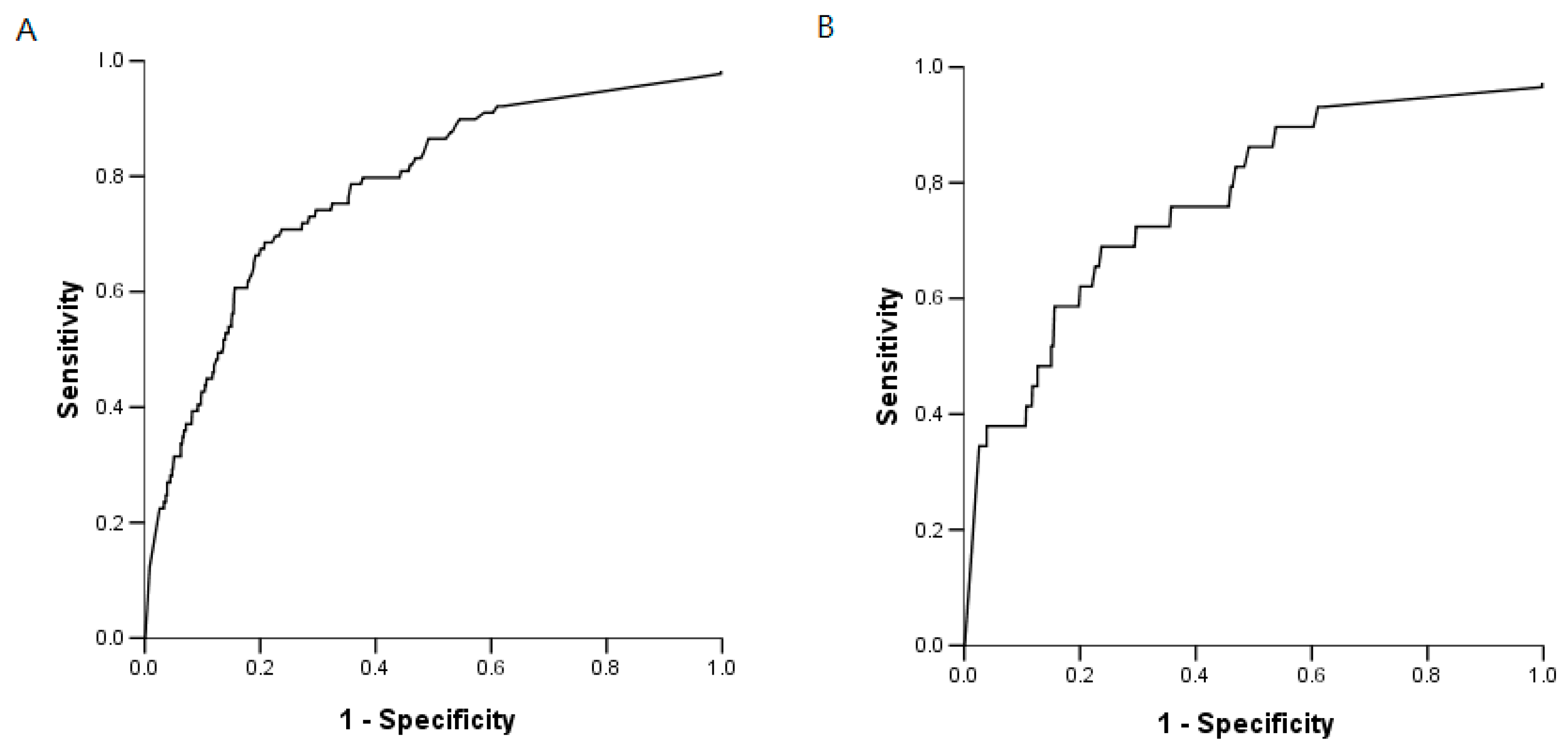
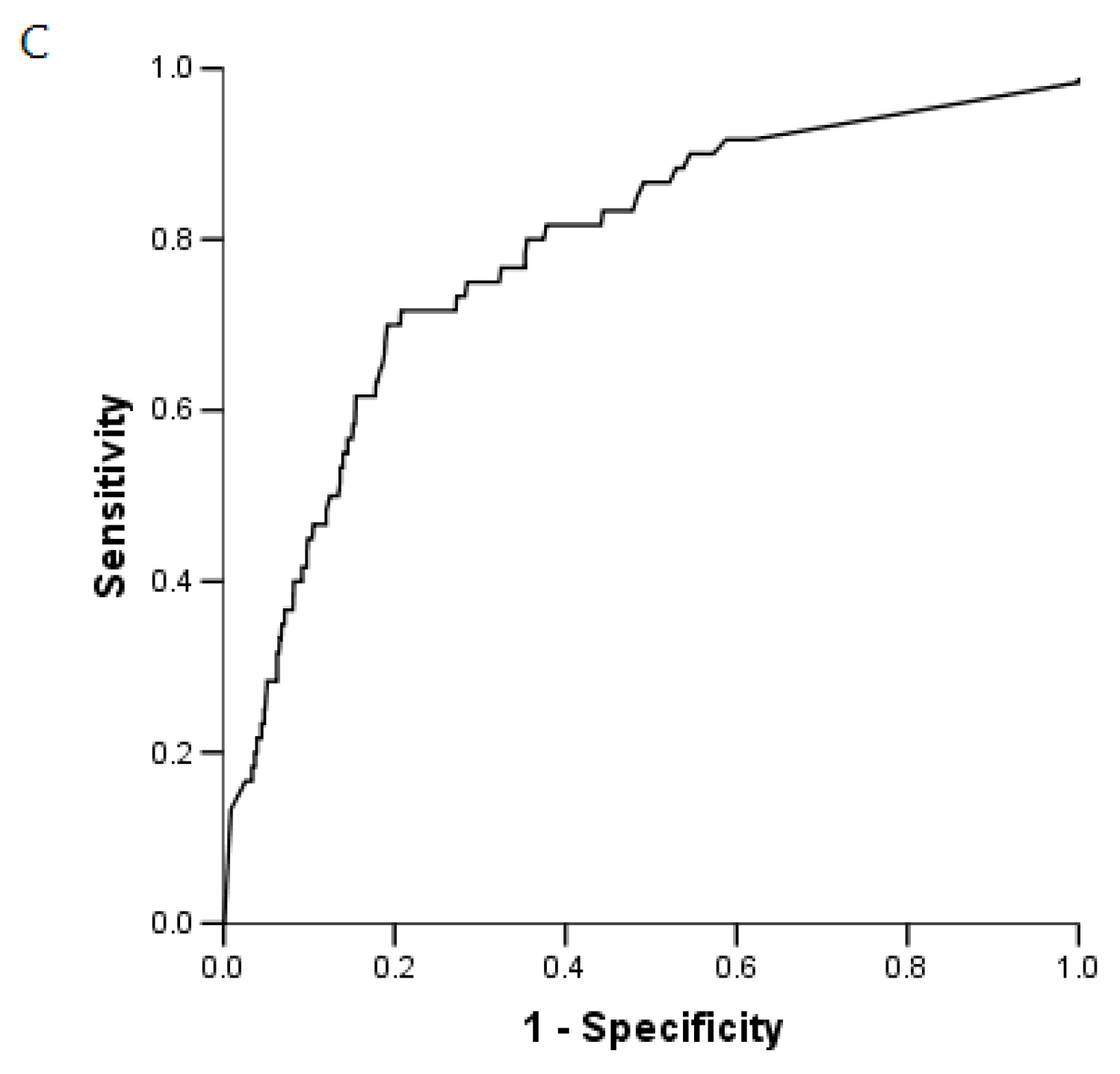
2.5. BG Analysis
2.6. IFI and BG
3. Discussion
4. Patients and Methods
4.1. Clinical and Biological Management
4.2. Antifungal Prophylaxis
4.3. Colonisation
4.4. Galactomannan
4.5. (1,3)-β-d-Glucan (BG) Assay
4.6. Definition
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| AUROC | Area Under Receiver Operating Characteristic |
| BG | (1,3)-β-d-glucan |
| CMV | Cytomegalovirus |
| EORTC/MSG | European Organization for the Research and Treatment of Cancer/Mycoses Study Group |
| IC | Invasive Candidiasis |
| ICU | Intensive Care Unit |
| IFI | Invasive Fungal Infection |
| IPA | Invasive Pulmonary Aspergillosis |
| LT | Liver Transplantation |
| MELD Score | Model for End-Stage Liver Disease |
| NPV | Negative Predictive Value |
| PjP | Pneumocystis jirovecii pneumonia |
| PPV | Positive Predictive Value |
| ROC | Receiver Operating Characteristic |
References
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Kontoyiannis, D.P. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Fishman, J.A.; Horn, D.; Anaissie, E.; Chang, C.H.; Olyaei, A.; Marr, K.A. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; He, X.S.; Chen, J.; Ouyang, B.; Zhu, X.F.; Chen, M.Y.; Chen, X.X. Fungal infection in patients after liver transplantation in years 2003 to 2012. Ann. Transplant. Q. Pol. Transplant. Soc. 2012, 17, 59–63. [Google Scholar]
- Grossi, P.A. Clinical aspects of invasive candidiasis in solid organ transplant recipients. Drugs 2009, 69, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Avery, R.K.; Munoz, P.; Pruett, T.L.; Alexander, B.; Jacobs, R.; Husain, S. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin. Infect. Dis. 2003, 36, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wen, T.F.; Mi, K.; Wang, C.; Yan, L.N.; Li, B. Analysis of infections in the first 3-month after living donor liver transplantation. World J. Gastroenterol. 2012, 18, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; Wang, S.H.; Liang, S.X.; Jiang, W.X.; Luo, D.D.; Huang, D.H. The screening performance of serum 1,3-β-d-glucan in patients with invasive fungal diseases: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0131602. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hang, J.P.; Zhang, L.; Wang, F.; Zhang, D.C.; Gong, F.H. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-d-glucan for invasive fungal infection: Focus on cutoff levels. J. Microbiol. Immunol. Infect. 2015, 48, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Sugiyama, D.; Kogata, Y.; Saegusa, J.; Sugimoto, T.; Kawano, S.; Kumagai, S. Diagnostic accuracy of serum 1,3-β-d-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: Systematic review and meta-analysis. J. Clin. Microbiol. 2012, 50, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Cruciani, M.; Mengoli, C.; Castagnola, E.; Lortholary, O.; Richardson, M.; Marchetti, O. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: A systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin. Infect. Dis. 2012, 54, 633–643. [Google Scholar] [PubMed]
- Karageorgopoulos, D.E.; Voulouman, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Falagas, M.E. β-d-Glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Sugawara, Y.; Kaneko, J.; Tamura, S.; Makuuchi, M. Preemptive treatment of fungal infection based on plasma (1→3) β-d-glucan levels after liver transplantation. Infection 2007, 35, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Winston, D.J.; Limaye, A.P.; Pelletier, S.; Safdar, N.; Morris, M.I.; Wheat, L.J. Performance characteristics of galactomannan and β-d-glucan in high-risk liver transplant recipients. Transplantation 2015, 99, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Levesque, E.; El Anbassi, S.; Sitterle, E.; Foulet, F.; Merle, J.C.; Botterel, F. Contribution of (1,3)-β-d-glucan to diagnosis of invasive candidiasis after liver transplantation. J. Clin. Microbiol. 2015, 53, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Hadley, S.; Huckabee, C.; Pappas, P.G.; Daly, J.; Rabkin, J.; Kauffman, C.A.; Karchmer, A.W. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl. Infect. Dis. 2009, 11, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.A.; Samore, M.H.; Roberts, M.S.; Luzzati, R.; Jenkins, R.L.; Lewis, W.D.; Karchmer, A.W. Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J. Infect. Dis. 1994, 170, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; Delva, V.; Ichaï, P.; Kassis, N.; Botterel, F.; Mihaila, L.; Samuel, D. Fungal infections after liver transplantation: Outcomes and risk factors revisited in the MELD era. Clin. Transplant. 2013, 27, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.P.; Husain, S. Fungal infections in solid organ transplantation. Med. Mycol. 2007, 45, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Winston, D.J.; Limaye, A.P.; Pelletier, S.; Safdar, N.; Morris, M.I.; Meneses, K.; Singh, N. Randomized, double-blind trial of anidulafungin versus fluconazole for prophylaxis of invasive fungal infections in high-risk liver transplant recipients. Am. J. Transplant. 2014, 14, 2758–2764. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; Pascher, A.; Cointault, O.; Laterre, P.F.; Cervera, C.; de Waele, J.J.; Phillips, S. Randomized trial of micafungin for the prevention of invasive fungal infection in high-risk liver transplant recipients. Clin. Infect. Dis. 2015, 60, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Fortún, J.; Muriel, A.; Martín-Dávila, P.; Montejo, M.; Len, O.; Torre-Cisneros, J.; Fresco, G. Caspofungin versus fluconazole as prophylaxis of invasive fungal infection in high-risk liver transplantation recipients: A propensity score analysis. Liver Transpl. 2016, 22, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Alexander, B.D.; Kett, D.H.; Vazquez, J.; Pappas, P.G.; Saeki, F.; Finkelman, M.A. Multicenter clinical evaluation of the (1→3) β-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 2005, 41, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, Z.; Mattiuzzi, G.; Estey, E.; Kantarjian, H.; Saeki, F.; Ridge, R.J.; Ostrosky-Zeichner, L. β-d-Glucan as a diagnostic adjunct for invasive fungal infections: Validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 2004, 39, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Racil, Z.; Kocmanova, I.; Lengerova, M.; Weinbergerova, B.; Buresova, L.; Toskova, M.; Mayer, J. Difficulties in using 1,3-β-d-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies—High frequency of false-positive results and their analysis. J. Med. Microbiol. 2010, 59, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Kubo, K.; Hamasaki, K.; Kanda, Y.; Nakao, A.; Kitamura, T.; Mimura, T. Influence of various hemodialysis membranes on the plasma (1→3)-β-d-glucan level. Kidney Int. 2001, 60, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Ohata, A.; Horiuchi, T.; Nagasawa, K.; Wakabayashi, T.; Tanaka, S. Positive (1→3)-β-d-glucan in blood components and release of (1→3)-β-d-glucan from depth-type membrane filters for blood processing. Transfusion 2002, 42, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Mennink-Kersten, M.A.; Warris, A.; Verweij, P.E. 1,3-β-d-Glucan in patients receiving intravenous amoxicillin-clavulanic acid. N. Engl. J. Med. 2006, 354, 2834–2835. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lincoln, L.; Marchillo, K.; Andes, D. β-1,3-Glucan as a test for central venous catheter biofilm infection. J. Infect. Dis. 2007, 195, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.F.; Sims, C.; Paetznick, V.; Rodriguez, J.; Finkelman, M.A.; Rex, J.H.; Ostrosky-Zeichner, L. Prospective survey of (1→3)-β-d-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J. Clin. Microbiol. 2011, 49, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Gavaldà, J.; Meije, Y.; Fortún, J.; Roilides, E.; Saliba, F.; Lortholary, O.; Cuenca-Estrella, M. Invasive fungal infections in SOT recipients. Clin. Microbiol. Infect. 2014, 20, 27–48. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Denning, D.W. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [PubMed]
| Variable | Liver Transplants n = 271 |
|---|---|
| Age (years) | 52.7 ± 12.7 |
| Gender M/F | 202/69 |
| Indication for liver transplantation | |
| Hepatocellular carcinoma | 85 (31) |
| Cirrhosis | 126 (46) |
| Fulminant hepatic failure | 17 (7) |
| Other | 43 (16) |
| Alcoholic liver disease | 130 (48) |
| Viral hepatitis (HCV and HBV) | 54 (20) |
| Other | 87 (32) |
| MELD score | 19.1 ± 11.4 |
| MELD < 20 | 164 (60) |
| MELD 20–30 | 47 (18) |
| MELD > 30 | 60 (22) |
| Antifungal prophylaxis n (%) | 157 (58) |
| Caspofungin, n (%) | 132 (84.1) |
| Micafungin, n (%) | 6 (3.8) |
| Fluconazole, n (%) | 18 (11.5) |
| Ampho B, n (%) | 1 (0.6) |
| duration of treatment (days) | 24.3 ± 18.5 |
| Risk factors for IFI | |
| MELD > 30 | 60 (22) |
| Fulminant hepatic failure | 17 (6.3) |
| Re-transplantation | 20 (7.5) |
| Graft type | |
| Whole cadaveric liver transplant | 246 (91) |
| Partial liver transplant (split) | 22 (8) |
| Domino | 3 (1) |
| Multi-organ transplantation (kidney/liver) | 14 (5) |
| >40 transfusions of blood products | 8 (3) |
| Renal Replacement therapy | 44 (16) |
| Early re-intervention after LT | 51 (19) |
| Choledocojejunostomy anastomosis (Roux-en-Y) | 38 (14) |
| Patient Number | Delay between LT and IC (days) | Localisation IC | Culture | Classification of IC | Values BG (pg/mL) | CI | Prophylactic Treatment (Delay before IC) | Curative Treatment | Outcome (after LT) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Intra-abdominal | C. glabrata | Proven | 201 | 0.5 | Caspofungin (45) | Voriconazole | Died 301 days |
| 252 | Candidemia | C. parapsilosis | Proven | 501 | 0.8 | Caspofungin (25) | Fluconazole | ||
| 2 | 65 | Intra-abdominal | C. glabrata | Proven | 501 | 0.8 | Caspofungin (10) | Voriconazole | Died 81 days |
| 3 | 26 | Intra-abdominal candidiasis | C. glabrata, C. albicans | Proven | 366 | 0.8 | - | Caspofungin | Died 91 days |
| 4 | 7 | Intra-abdominal | C. albicans | Proven | 360 | 0.8 | Caspofungin (7) | Fluconazole | Died 47 days |
| 5 | 8 | Candidemia | C. albicans | Proven | 501 | 0.6 | Fluconazole (8) | Fluconazole | Alive 692 days |
| 6 | 7 | Intra-abdominal/biliary abscess | C. albicans | Proven | 501 | 0.8 | Caspofungin (7) | Fluconazole | Alive 677 days |
| 7 | 6 | Candidemia | C. albicans | Proven | 170 | 0.6 | Caspofungin (6) | Fluconazole | Alive 438 days |
| 8 | 22 | Intra-abdominal | C. krusei | Proven | 320 | 0.2 | Caspofungin (22) | Caspofungin | Alive 317 days |
| 9 | 62 | Candidemia | C. orthopsilosis | Proven | 173 | 0.5 | - | Liposomal Amphotericin B | Alive 177 days |
| 10 | 10 | Intra-abdominal | C. krusei, C. albicans | Proven | 209 | 0.6 | Caspofungin (10) | Liposomal Amphotericin B | Alive 49 days |
| 11 | 12 | Intra-abdominal and candidemia | C. glabrata | Proven | 246 | 0.6 | Caspofungin (10) | Caspofungin | Died 280 days |
| 86 | abscess and biliary infection | C. glabrata, C. albicans, C. tropicalis | Probable | 501 | 0.5 | - | Caspofungin |
| Patient Number | Time to Onset after LT (days) | Underlying Diseases | IPA Classification | BAL Direct Examination | Culture | GM in Serum (Index) | GM in BAL (Index) | BG Values (pg/mL) | Prophylactic Treatment (Delay) | Curative Treatment | Outcome (after LT) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 5 | Cryptogenetic cirrhosis | Probable | NA | A. fumigatus | + (0.602) | NA | 207 | Caspofungin (5) | Voriconazole | Alive at 1197 days |
| 13 | 44 | Acute liver failure (autoimmune hepatitis) | Probable | - | A. fumigatus | − (0.125) | + (1.25) | 501 | Caspofungin (35) | Voriconazole | Alive at 1197 days |
| 14 | 45 | HCC/VHB cirrhosis | Probable | - | A. fumigatus | + (>6) | + (5.58) | 198 | Caspofungin (9) | Voriconazole | Died 61 days |
| 15 | 7 | Acute liver failure (toxic hepatitis) | Probable | NA | A. fumigatus | + (1.39) | + (1.85) | 219 | Caspofungin (7) | Voriconazole | Alive at 599 days |
| 16 | 5 | Acute liver failure Reactivation of VHB infection | Probable | - | A. fumigatus | − (0.06) | − (0.13) | 501 | Caspofungin (5) | Voriconazole | Alive at 542 days |
| 17 | 32 | Re-LT Chronic graft rejection | Probable | - | A. fumigatus | + (2.361) | NA | 42 | Caspofungin (31) | Voriconazole | Alive at 41 days after LT |
| 18 | 25 | VHB/VHC cirrhosis | Probable | - | A. fumigatus | − (0.157) | + (1.6) | 170 | Caspofungin (19) | Voriconazole | Died 26 days |
| 2 | 55 | Acute liver failure (toxic hepatitis) | Probable | - | A. fumigatus | + (5.30) | + (5.68) | 423 | Caspofungin (35) | Voriconazole | Died 81 days |
| 19 | 22 | Acute liver failure Reactivation of VHB infection | Proven Septic arthritis of the hip | Scedosporium sp. | + (2.83) | NA | 501 | Micafungin (22) | Surgery Caspofungin + voriconazole | Alive at 599 days |
| IFI | Patient Number | Day 7 | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|---|
| IC | 1 | 201 | 147 | NA | NA | 41 | 44 |
| 1 | 501 | 356 | 393 | 501 | 501 | 446 | |
| 2 | 234 | 423 | 501 | 501 | 501 | NA | |
| 3 | NA | 36 | 366 | NA | 50 | 77 | |
| 4 | 360 | 464 | 501 | NA | 31 | NA | |
| 5 | NA | 501 | 269 | 269 | 191 | 221 | |
| 6 | 324 | 348 | 14 | 501 | 323 | 192 | |
| 7 | 106 | 153 | 170 | 110 | 90 | 51 | |
| 8 | 232 | NA | 320 | 311 | NA | NA | |
| 9 | 31 | 151 | 173 | 76 | 140 | NA | |
| 10 | 31 | 209 | 148 | NA | NA | NA | |
| 11 | 170 | 31 | 58 | 180 | NA | 246 | |
| 11 | 187 | 501 | NA | NA | 293 | 170 | |
| IPA | 12 | 143 | 207 | 53 | 55 | NA | NA |
| 13 | 501 | NA | 174 | NA | NA | NA | |
| 14 | 198 | 169 | NA | NA | NA | 30 | |
| 15 | NA | 219 | 128 | 50 | NA | NA | |
| 16 | 501 | 501 | 501 | NA | 501 | 501 | |
| 17 | 31 | 33 | 42 | NA | NA | NA | |
| 18 | 75 | 170 | NA | NA | NA | NA | |
| 2 | 423 | 131 | NA | NA | NA | 99 | |
| Scedosporium sp. | 19 | 423 | 501 | 501 | 501 | NA | NA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levesque, E.; Rizk, F.; Noorah, Z.; Aït-Ammar, N.; Cordonnier-Jourdin, C.; El Anbassi, S.; Bonnal, C.; Azoulay, D.; Merle, J.-C.; Botterel, F. Detection of (1,3)-β-d-Glucan for the Diagnosis of Invasive Fungal Infection in Liver Transplant Recipients. Int. J. Mol. Sci. 2017, 18, 862. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040862
Levesque E, Rizk F, Noorah Z, Aït-Ammar N, Cordonnier-Jourdin C, El Anbassi S, Bonnal C, Azoulay D, Merle J-C, Botterel F. Detection of (1,3)-β-d-Glucan for the Diagnosis of Invasive Fungal Infection in Liver Transplant Recipients. International Journal of Molecular Sciences. 2017; 18(4):862. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040862
Chicago/Turabian StyleLevesque, Eric, Fadi Rizk, Zaid Noorah, Nawel Aït-Ammar, Catherine Cordonnier-Jourdin, Sarra El Anbassi, Christine Bonnal, Daniel Azoulay, Jean-Claude Merle, and Françoise Botterel. 2017. "Detection of (1,3)-β-d-Glucan for the Diagnosis of Invasive Fungal Infection in Liver Transplant Recipients" International Journal of Molecular Sciences 18, no. 4: 862. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040862





