ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish
Abstract
:1. Introduction
2. Results
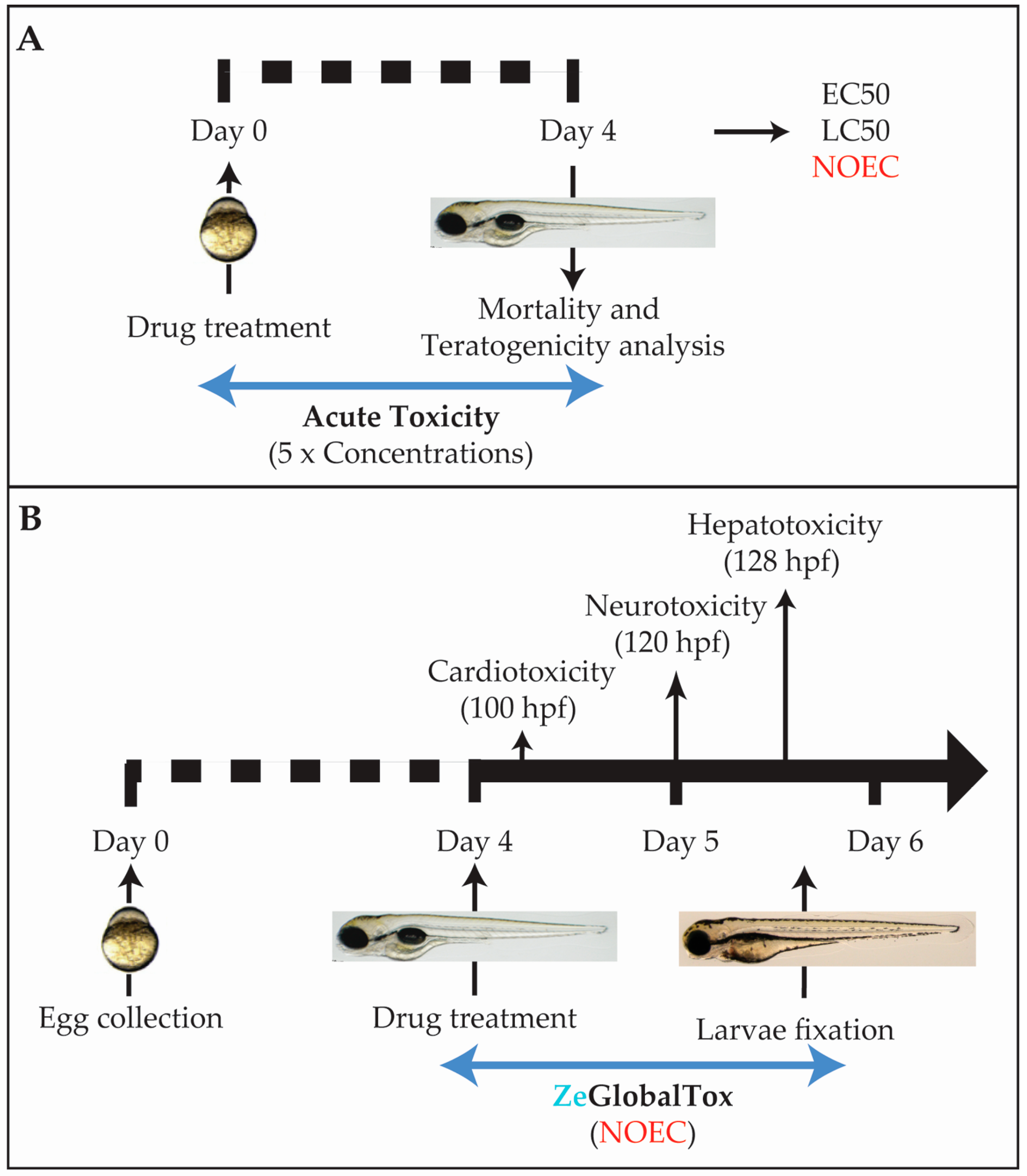
2.1. Experimental Workframe
2.2. Test Compounds
2.3. Acute Tox Analysis
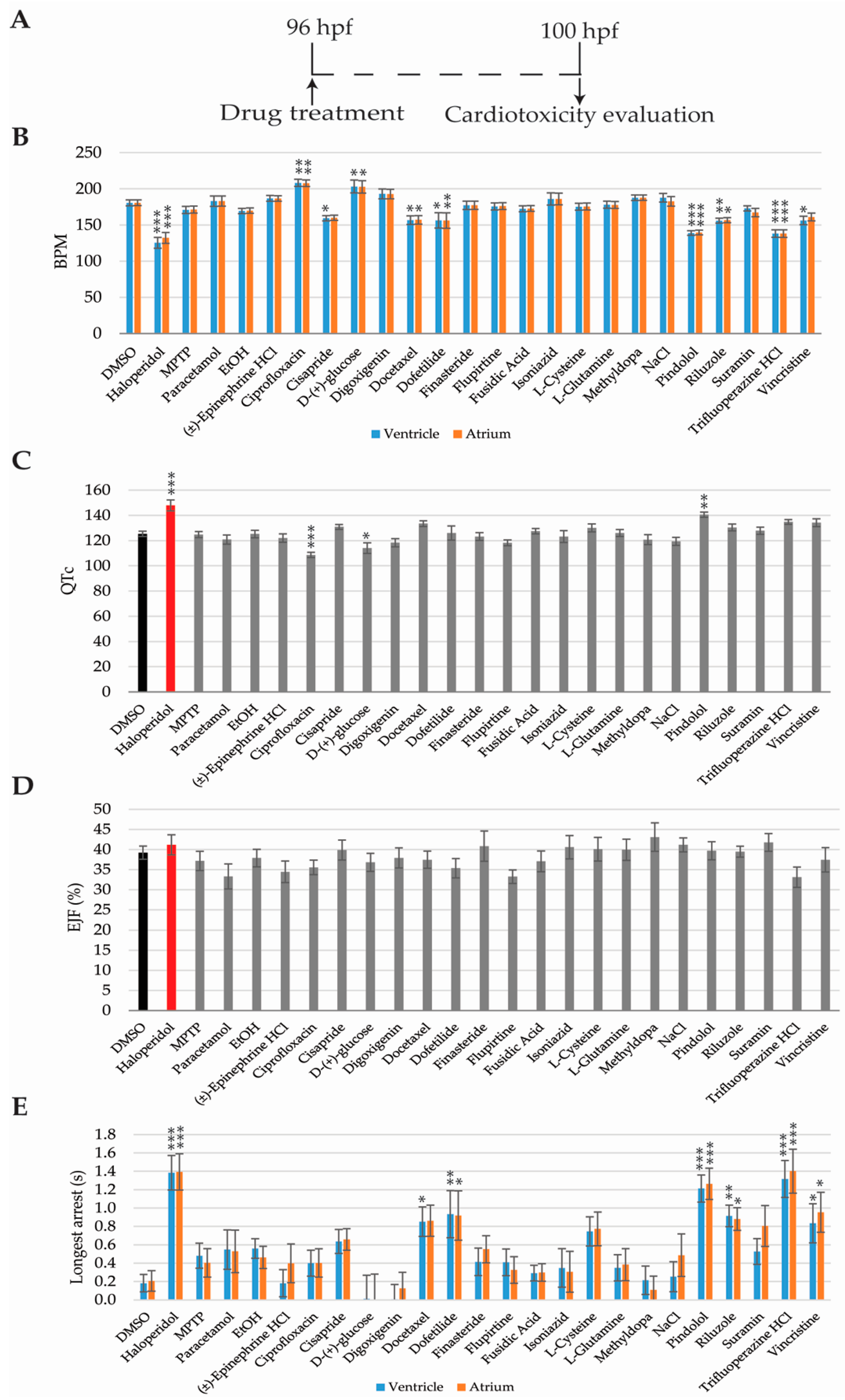
2.4. Cardiotoxicity Analysis
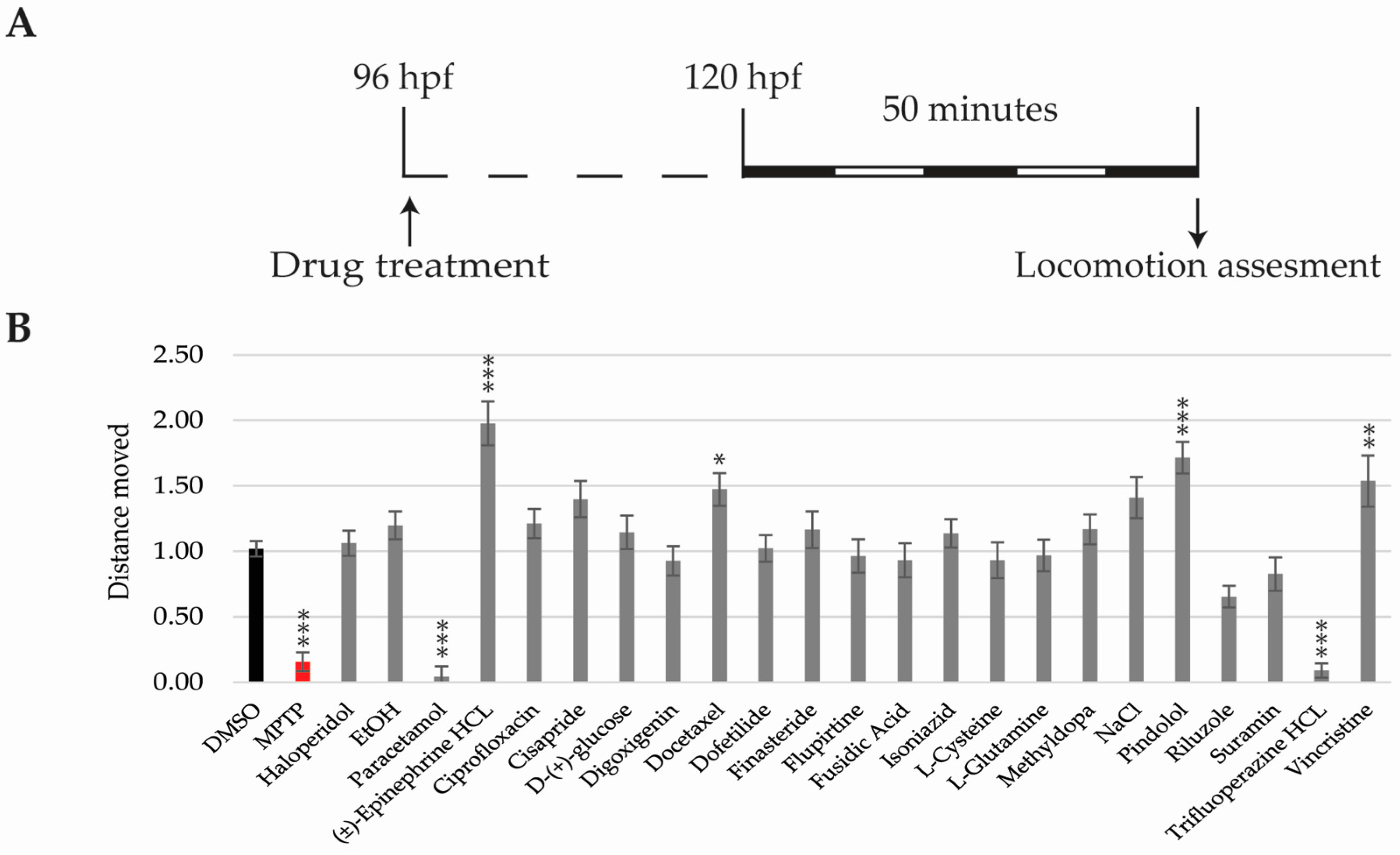
2.5. Locomotor Activity Analysis
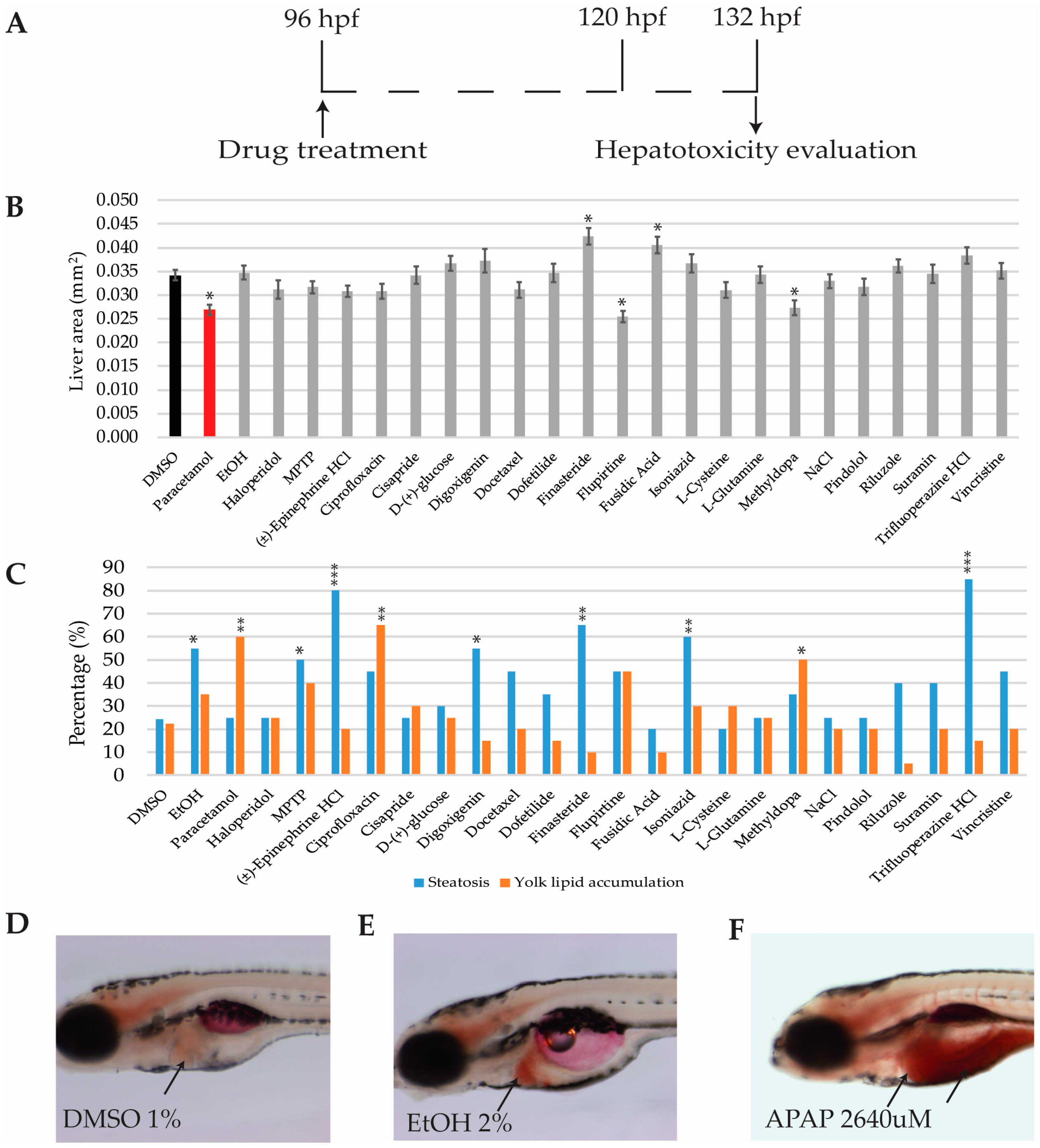
2.6. Hepatotoxicity Analysis
2.6.1. Hepatomegaly and Liver Necrosis Evaluation
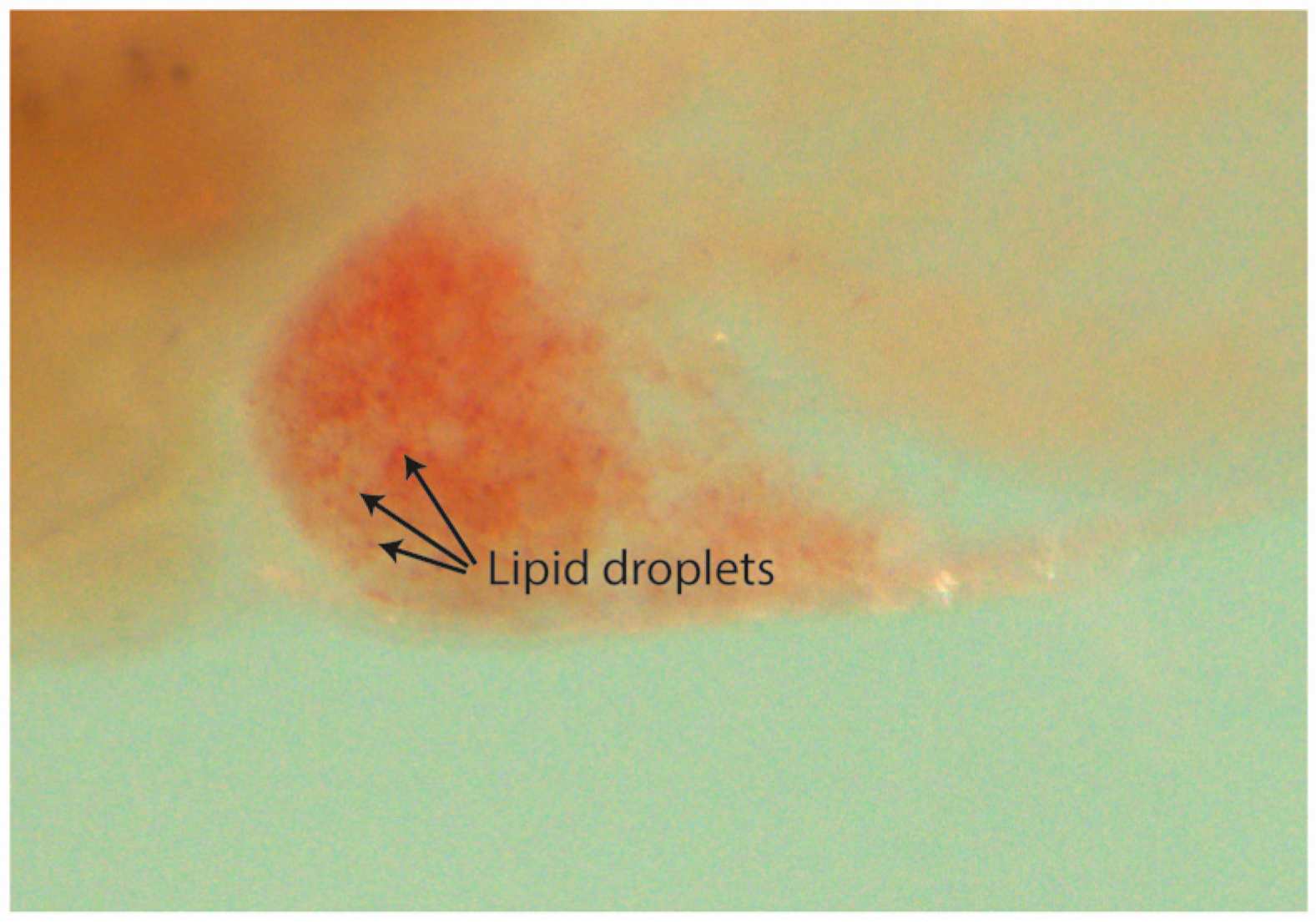
2.6.2. Steatosis and Yolk Lipid Accumulation Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Zebrafish Maintenance
4.3. Drug Exposure Conditions
4.4. Cardiotoxicity Evaluation in Zebrafish Larvae
4.5. Neurotoxicity Evaluation in Zebrafish Larvae
4.6. Hepatoxicity Evaluation in Zebrafish
4.6.1. Liver Area Analysis
4.6.2. Oil Red O Staining
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
| hpf | Hours post fertilization |
References
- Blomme, E.A.G.; Will, Y. Toxicology Strategies for Drug Discovery: Present and Future. Chem. Res. Toxicol. 2016, 29, 473–504. [Google Scholar] [CrossRef] [PubMed]
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Hornberg, J.J.; Laursen, M.; Brenden, N.; Persson, M.; Thougaard, A.V.; Toft, D.B.; Mow, T. Exploratory toxicology as an integrated part of drug discovery. Part I: Why and how. Drug Discov. Today 2014, 19, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.R.; Chen, S.-J.; He, A.; Truong, A.; Bhaskaran, V.; Nelson, D.M.; Dambach, D.M.; Lehman-McKeeman, L.D.; Car, B.D. A retrospective analysis of toxicogenomics in the safety assessment of drug candidates. Toxicol. Pathol. 2007, 35, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.; Laggner, C.; Langer, T. Why Drugs Fail—A Study on Side Effects in New Chemical Entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.; Brown, A.J.; Hamon, J.; Jarolimek, W.; Sridhar, A.; Waldron, G.; Whitebread, S. Reducing safety-related drug attrition: The use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 2012, 11, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An Emerging Model System for Human Disease and Drug Discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Raldúa, D.; Piña, B. In vivo zebrafish assays for analyzing drug toxicity. Expert Opin. Drug Metab. Toxicol. 2014, 10, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Rennekamp, A.J.; Peterson, R.T. 15 Years of Zebrafish Chemical Screening. Curr. Opin. Chem. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Union Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 33–79. [Google Scholar]
- Halder, M.; Léonard, M.; Iguchi, T.; Oris, J.T.; Ryder, K.; Belanger, S.E.; Braunbeck, T.A.; Embry, M.R.; Whale, G.; Norberg-King, T.; et al. Regulatory aspects on the use of fish embryos in environmental toxicology. Integr. Environ. Assess. Manag. 2010, 6, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Embry, M.R.; Belanger, S.E.; Braunbeck, T.A.; Galay-Burgos, M.; Halder, M.; Hinton, D.E.; Léonard, M.A.; Lillicrap, A.; Norberg-King, T.; Whale, G. The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquat. Toxicol. 2010, 97, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Jones, I.L.; Ellinor, P.T.; Macrae, C.A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H269–H273. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.S.; Dóró, É.; Magyary, I.; Egginton, S.; Sík, A.; Müller, F. Optimisation of Embryonic and Larval ECG Measurement in Zebrafish for Quantifying the Effect of QT Prolonging Drugs. PLoS ONE 2013, 8, e60552. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.; Libourel, P.-A.; Hetheridge, M.J.; Cumming, R.I.; Sutcliffe, T.P.; Goonesinghe, A.C.; Ball, J.S.; Owen, S.F.; Chomis, Y.; Winter, M.J. A multi-endpoint in vivo larval zebrafish (Danio rerio) model for the assessment of integrated cardiovascular function. J. Pharmacol. Toxicol. Methods 2014, 69, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.C.; Williams, A.C.; Markey, S.P.; Ebert, M.H.; Caine, E.D.; Reichert, C.M.; Kopin, I.J. Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979, 1, 249–254. [Google Scholar] [CrossRef]
- Sarath Babu, N.; Murthy, C.L.N.; Kakara, S.; Sharma, R.; Brahmendra Swamy, C.V.; Idris, M.M. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease in zebrafish. Proteomics 2016, 16, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of Acetaminophen-Induced Liver Necrosis. Handb. Exp. Pharmacol. 2011, 1–34. [Google Scholar]
- Lieber, C.S. Metabolism of ethanol and associated hepatotoxicity. Drug Alcohol Rev. 1991, 10, 175–202. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.L.; Passeri, M.; Sadler, K.C. Drinks like a fish: Using zebrafish to understand alcoholic liver disease. Alcohol Clin. Exp. Res. 2011, 35, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Tsedensodnom, O.; Vacaru, A.M.; Howarth, D.L.; Yin, C.; Sadler, K.C. Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis. Model. Mech. 2013, 6, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart, A.D.; Tucker, C.S.; Del Pozo, J.; Dear, J.W. Zebrafish as model organisms for studying drug induced liver injury. Br. J. Clin. Pharmacol. 2014, 78, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Mesens, N.; Crawford, A.D.; Menke, A.; Hung, P.D.; Van Goethem, F.; Nuyts, R.; Hansen, E.; Wolterbeek, A.; Van Gompel, J.; De Witte, P.; et al. Are zebrafish larvae suitable for assessing the hepatotoxicity potential of drug candidates? J. Appl. Toxicol. 2015, 35, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wei, W.; Gu, W.; Huang, P.; Ren, X.; Zhang, Z.; Zhu, Z.; Lin, S.; Zhang, B. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev. Biol. 2008, 314, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, V.; Cline, H.T. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J. Neurophysiol. 2008, 100, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, K. Fusidic acid adverse drug reactions. Int. J. Antimicrob. Agents 1999, 12 (Suppl. S2), S3–S9. [Google Scholar] [CrossRef]
- Leitner, J.M.; Graninger, W.; Thalhammer, F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical. Infection 2010, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, M.A.; Reyno, L.M.; Jodrell, D.I.; Sinibaldi, V.J.; Tkaczuk, K.H.; Sridhara, R.; Zuhowski, E.G.; Lowitt, M.H.; Jacobs, S.C.; Egorin, M.J. Suramin, an active drug for prostate cancer: Interim observations in a phase I trial. J. Natl. Cancer Inst. 1993, 85, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Reed, E.; Sartor, O.; Dahut, W.; Figg, W.D. Suramin’s development: What did we learn? Investig. New Drug 2002, 20, 209–219. [Google Scholar] [CrossRef]
- Leong, I.U.S.; Skinner, J.R.; Shelling, A.N.; Love, D.R. Zebrafish as a model for long QT syndrome: The evidence and the means of manipulating zebrafish gene expression. Acta Physiol. 2010, 199, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.S.; Wu, Q.; Xiao, C.L.; Chang, N.N.; Wang, X.; Lei, L.; Zhu, X.; Xiong, J.W. Overlapping Cardiac Programs in Heart Development and Regeneration. J. Genet. Genom. 2012, 39, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.G.; Scott, J.G. Zebrafish: A Model System for the Study of Eye Genetics. Prog. Retin. Eye Res. 2012, 100, 130–134. [Google Scholar]
- Colwill, R.M.; Creton, R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav. Process. 2011, 86, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Anichtchik, O.V.; Kaslin, J.; Peitsaro, N.; Scheinin, M.; Panula, P. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. 2004, 88, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, B.J.; Pack, M. Zebrafish Models of Human Liver Development and Disease. Compr. Physiol. 2013, 3, 1213–1230. [Google Scholar] [PubMed]
- Chu, J.; Kirsten, C.; Sadler, A. New School in Liver Development: Lessons from Zebrafish. Hepatology 2009, 50, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Babu, I.R.; Vedder, L.M.; Lord, A.M.; Wishnok, J.S.; Tannenbaum, S.R.; Zon, L.I.; Goessling, W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc. Natl. Acad. Sci. USA 2010, 107, 17315–17320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, C.; Gong, Z. Development of a convenient in vivo hepatotoxin assay using a transgenic zebrafish line with liver-specific dsred expression. PLoS ONE 2014, 9, e91874. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Alimov, A.P.; Rilo, H.L.; Jandacek, R.J.; Woollett, L.A.; Penberthy, W.T. A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr. Metab. 2008, 5, 1743–7075. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Guo, S.Y.; Zhu, F.; Zhu, J.J.; Chen, Y.X.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Xuan, Y.X.; Li, C.Q. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J. Pharmacol. Toxicol. Methods 2013, 67, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Gomez-Lechon, M.J. Drug-induced Liver Steatosis and Phospholipidosis: Cell-Based Assays for Screening of Drug Candidates. Curr. Drug Metab. 2012, 13, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.A.; Sagartz, J.E.; Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 2007, 6, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Passeri, M.J. Hepatic Steatosis in Response to Acute Alcohol Exposure in Zebrafish requires Srebp Activation. Hepatology 2010, 49, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.G.; Milan, D.J.; Grande, E.J.; Rottbauer, W.; MacRae, C.A.; Fishman, M.C. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat. Chem. Biol. 2005, 1, 263–264. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A. Cardiac Arrhythmia: In vivo screening in the zebrafish to overcome complexity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- De Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing. A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Legradi, J.; el Abdellaoui, N.; van Pomeren, M.; Legler, J. Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity. Environ. Sci. Pollut. Res. 2014, 22, 16277–16289. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Mesens, N.; Steemans, M.; Xu, J.J.; Aleo, M.D. Comparisons between in vitro whole cell imaging and in vivo zebrafish-based approaches for identifying potential human hepatotoxicants earlier in pharmaceutical development. Drug Metab. Rev. 2012, 44, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Driessen, M.; Kienhuis, A.S.; Pennings, J.L.A.; Pronk, T.E.; Van De Brandhof, E.J.; Roodbergen, M.; Spaink, H.P.; Van De Water, B.; Van Der Ven, L.T.M. Exploring the zebrafish embryo as an alternative model for the evaluation of liver toxicity by histopathology and expression profiling. Arch. Toxicol. 2013, 87, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Liu, A.; Chen, F.; Yang, J.; Dai, R. Validation of visualized transgenic zebrafish as a high throughput model to assay bradycardia related cardio toxicity risk candidates. J. Appl. Toxicol. 2012, 32, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Nappo, F.; De Angelis, L.; Siniscalchi, M.; Rossi, F.; Giugliano, D. The effect of acute hyperglycaemia on QTc duration in healthy man. Diabetologia 2000, 43, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.R.; Hwang, S.; Coady, S.; Michael, J.; D’Agostino, R.B.S.; Savage, P.J.; Levy, D.; Fox, C.S. Trends in All-Cause and Cardiovascular Disease Mortality among Women and Men with and without Diabetes in the Framingham Heart Study, 1950–2005. Circulation 2009, 119, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. Diabetes: Advances in Diagnosis and Treatment. JAMA 2015, 314, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Daviaud, F.; Dumas, F.; Demars, N.; Geri, G.; Bouglé, A.; Morichau-Beauchant, T.; Nguyen, Y.L.; Bougouin, W.; Pène, F.; Charpentier, J.; et al. Blood glucose level and outcome after cardiac arrest: Insights from a large registry in the hypothermia era. Intensive Care Med. 2014, 40, 855–862. [Google Scholar] [PubMed]
- Du, M.; Fang, C.; Qiu, L.; Dong, S.; Zhang, X.; Yan, C. Diastereoisomer-specific effects of hexabromocyclododecanes on hepatic aryl hydrocarbon receptors and cytochrome P450s in zebrafish (Danio rerio). Chemosphere 2015, 132, 24–31. [Google Scholar] [PubMed]
- Timme-Laragy, A.; Cockman, C.; Matson, C.; Di Giulio, R. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat. Toxicol. 2007, 454, 42–54. [Google Scholar]
- Klüver, N.; Ortmann, J.; Paschke, H.; Renner, P.; Ritter, A.P.; Scholz, S. Transient overexpression of adh8a increases allyl alcohol toxicity in zebrafish embryos. PLoS ONE 2014, 9, 1–8. [Google Scholar]
- Liu, L.Y.; Fox, C.S.; North, T.E.; Goessling, W. Functional validation of GWAS gene candidates for abnormal liver function during zebrafish liver development. Dis. Model. Mech. 2013, 6, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Arner, P.; Yki-Jarvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Deibert, D.C.; Defronzo, R.A. Epinephrine-induced insulin resistance in man. J. Clin. Investig. 1980, 65, 717–721. [Google Scholar] [PubMed]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed]
- Martínez de Guzmán, M.; Martínez-Crespo, J.J. Finasteride-induced hepatitis. Farm. Hosp. 2006, 30, 385. [Google Scholar] [PubMed]
- Frey, W.A.; Vallee, B.L. Digitalis metabolism and human liver alcohol dehydrogenase. Proc. Natl. Acad. Sci. USA 1980, 77, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Di Monte, D.; Ekstrom, G.; Shinka, T.; Smith, M.T.; Trevor, A.J.; Castagnoli, N.J. Role of 1-methyl-4-phenylpyridinium ion formation and accumulation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity to isolated hepatocytes. Chem. Biol. Interact. 1987, 62, 105–116. [Google Scholar] [CrossRef]
- Ohta, S. Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in perfused rat liver: Involvement of hepatic aldehyde oxidase as a detoxification enzyme. Drug Metab. Dispos. 2000, 28, 538–543. [Google Scholar]
- Bisgin, H. Toward predictive models for drug-induced liver injury in humans: Are we there yet? Biomark. Med. 2014, 8, 201–213. [Google Scholar]
- Dong, P.D.S.; Munson, C.A.; Norton, W.; Crosnier, C.; Pan, X.; Gong, Z.; Neumann, C.J.; Stainier, D.Y.R. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat. Genet. 2007, 39, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Korzh, S.; Li, Z.; Mudumana, S.P.; Korzh, V.; Jiang, Y.J.; Lin, S.; Gong, Z. Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp. Cell Res. 2006, 312, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.L.; Yin, C.; Yeh, K.; Sadler, K.C. Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae. Zebrafish 2013, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Templeton, G.F. A two-step approach for transforming continuous variables to normal: Implications and recommendations for IS research. Commun. Assoc. Inf. Syst. 2011, 28, 41–58. [Google Scholar]


| Drug | Cardiotoxicity | Neurotoxicity | Hepatotoxicity |
|---|---|---|---|
| Acetaminophen | Toxic 1,2,3 | Toxic 2,3 | Toxic 1,2,3 [24] |
| Ethanol | Toxic 1 | Toxic 1 | Toxic [26,27] |
| Haloperidol | Toxic 1,2,3 [19,20,21] | Toxic 1,2,3 | Safe |
| MPTP | A | Toxic [22,30,31] | A |
| (±)-Epinephrine HCL | Toxic 1,2,3 | Toxic 1,2,3 | Safe |
| Ciprofloxacin | Toxic 1,2,3 | Toxic 1,2,3 | Toxic 1,2,3 |
| Cisapride | Toxic 1,3 | Safe | Safe |
| d-(+)-glucose | Safe | Safe | Safe |
| Digoxigenin | Toxic 1 | Safe | Safe |
| Docetaxel | Toxic 2,3 | Toxic 1,2,3 | Toxic 1,2,3 |
| Dofetilide | Toxic 1,2,3 | Safe | Safe |
| Finasteride | Safe | Safe | Safe |
| Flupirtine | Safe | Safe | Toxic 4 |
| Fusidic Acid | Safe | Safe | Toxic [32,33] |
| Isoniazid | Safe | Toxic 1,2,3 | Toxic 1,2,3 |
| l-Cysteine | Safe | Safe | Safe |
| l-Glutamine | Safe | Safe | Safe |
| Methyldopa | Safe | Toxic 1,3 | Toxic 1,2,3 |
| NaCl | Safe | Safe | Safe |
| Pindolol | Toxic 1,2,3 | Toxic 2,3 | Safe |
| Riluzole | Toxic 2,3 | Safe | Toxic 2,3 |
| Suramin | Safe | Toxic [34,35] | Safe |
| Trifluoperazine HCL | Toxic 1,2,3 | Toxic 1,2,3 | Toxic 1,2,3 |
| Vincristine | Toxic 1,2,3 | Toxic 1,2,3 | Safe |
| Drug | 96 hpf NOEC (µM) | 96 hpf LOEC (µM) | 96 hpf LC50 (µM) |
|---|---|---|---|
| DEAB | 1.00 | 10.00 | 185.99 |
| (±)-Epinephrine hydrochloride | 1000.00 | N.A. | 3.11 × 1010 |
| Ciprofloxacin | 1000.00 | N.A. | 7.01 × 109 |
| Cisapride | 1000.00 | N.A. | 2935.74 |
| d-(+)-glucose | 1000.00 | N.A. | N.A |
| Digoxigenin | 100.00 | 1000.00 | N.A |
| Docetaxel | 10.00 | 100.00 | N.A |
| Dofetilide | 10.00 | 100.00 | N.A |
| Finasteride | 10.00 | 100.00 | 31.62 |
| Flupirtine | 10.00 | 100.00 | 136.05 |
| Fusidic Acid | 10.00 | 100.00 | 124.15 |
| Isoniazid | 1000.00 | N.A. | N.A |
| l-Cysteine | 1000.00 | N.A. | N.A |
| l-Glutamine | 1000.00 | N.A. | N.A |
| Methyldopa | 1000.00 | N.A. | N.A |
| NaCl | 1000.00 | N.A. | N.A |
| Pindolol | 100 | 1000 | 6608.09 |
| Riluzole | 1 | 10 | 13.71 |
| Suramin | 100 | 1000 | 196.56 |
| Trifluoperazine hydrochloride | 10 | 100 | 31.62 |
| Vincristine | 10 | 100 | 31.62 |
| Drug | Cardiotoxicity | Neurotoxicity | Hepatotoxicity | |
|---|---|---|---|---|
| (±)-Epinephrine HCL | FN | TP | FP | |
| Ciprofloxacin | TP | FN | TP | |
| Cisapride | TP | TN | TN | |
| d-(+)-glucose | FP | TN | TN | |
| Digoxigenin | FN | TN | FP | |
| Docetaxel | TP | TP | FN | |
| Dofetilide | TP | TN | TN | |
| Finasteride | TN | TN | FP | |
| Flupirtine | TN | TN | TP | |
| Fusidic Acid | TN | TN | TP | |
| Isoniazid | TN | FN | TP | |
| l-Cysteine | TN | TN | TN | |
| l-Glutamine | TN | TN | TN | |
| Methyldopa | TN | FN | TP | |
| NaCl | TN | TN | TN | |
| Pindolol | TP | TP | TN | |
| Riluzole | TP | TN | FN | |
| Suramin | TN | FN | TN | |
| Trifluoperazine HCL | TP | TP | TP | |
| Vincristine | TP | TP | TN | |
| Acetaminophen | FN | TP | TP | |
| Ethanol | FN | FN | TP | |
| Haloperidol | TP | FN | TN | |
| MPTP | - | TP | - | ZeGlobalTox |
| Specificity | 90% | 100% | 77% | 89% |
| Sensitivity | 69% | 54% | 80% | 68% |
| Accuracy | 78% | 75% | 82% | 78% |
| PPV | 90% | 100% | 73% | 88% |
| NPV | 69% | 65% | 83% | 72% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornet, C.; Calzolari, S.; Miñana-Prieto, R.; Dyballa, S.; Van Doornmalen, E.; Rutjes, H.; Savy, T.; D’Amico, D.; Terriente, J. ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. Int. J. Mol. Sci. 2017, 18, 864. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040864
Cornet C, Calzolari S, Miñana-Prieto R, Dyballa S, Van Doornmalen E, Rutjes H, Savy T, D’Amico D, Terriente J. ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. International Journal of Molecular Sciences. 2017; 18(4):864. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040864
Chicago/Turabian StyleCornet, Carles, Simone Calzolari, Rafael Miñana-Prieto, Sylvia Dyballa, Els Van Doornmalen, Helma Rutjes, Thierry Savy, Davide D’Amico, and Javier Terriente. 2017. "ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish" International Journal of Molecular Sciences 18, no. 4: 864. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040864







