The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract
Abstract
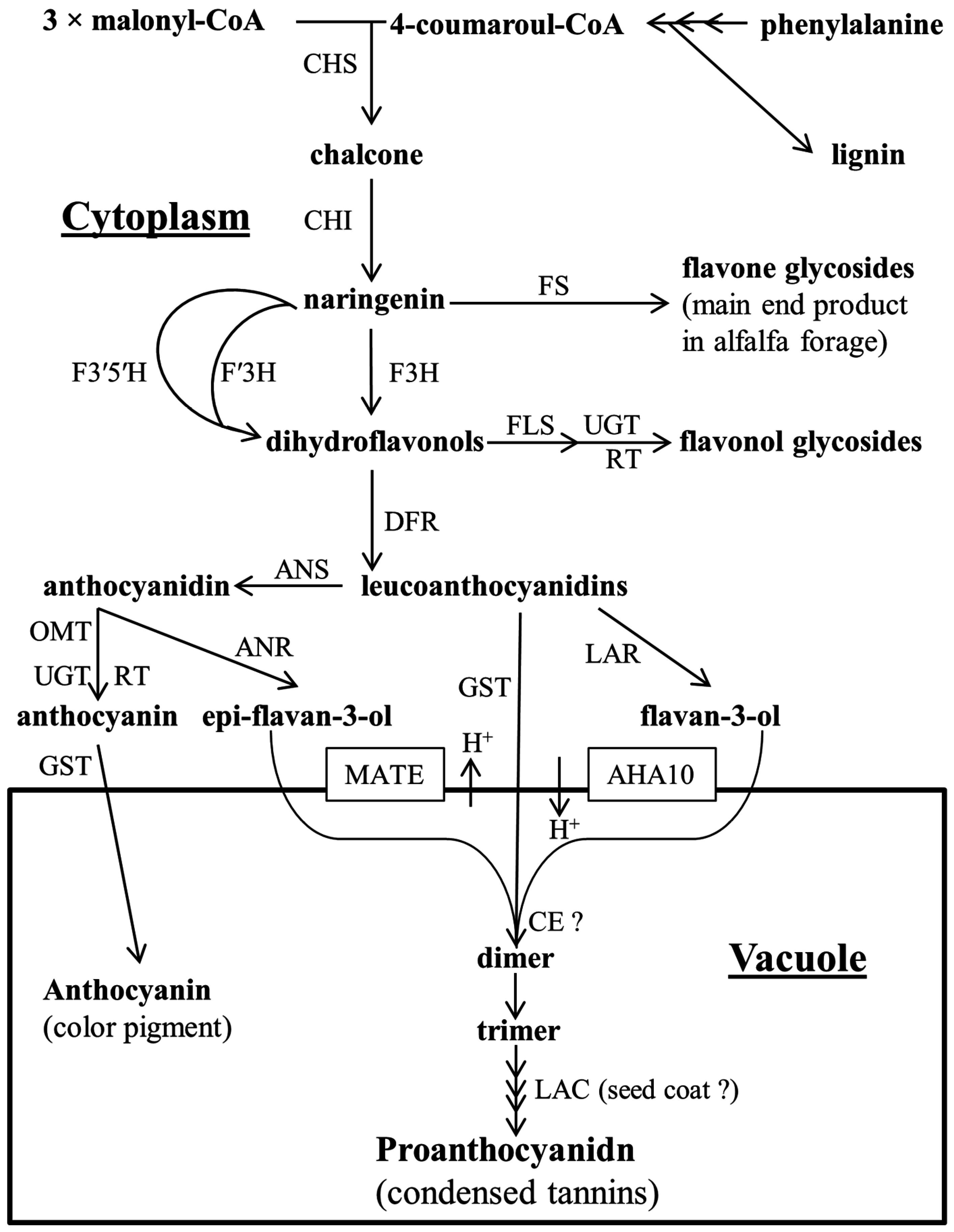
:1. General Introduction
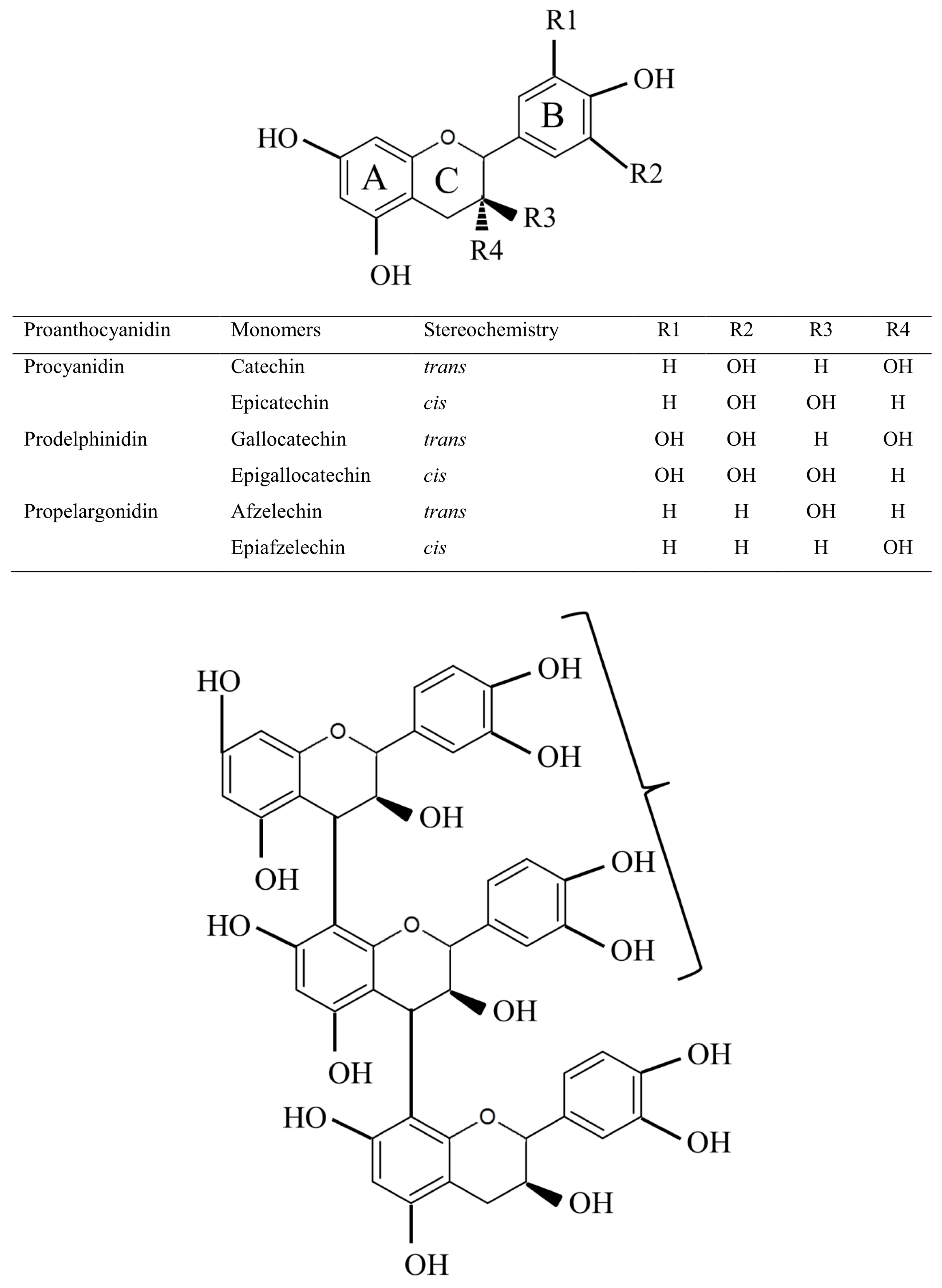
2. Proanthocyanidin Synthesis and Structure
3. Occurrence of Proanthocyanidin in Temperate/Prairie Forages
4. Proanthocyanidin Release from the Plant
5. Protein Precipitating Capacity as Affected by Proanthocyanidin Characteristics
6. Protein Precipitating Capacity of Proanthocyanidins as Affected by Protein Characteristics
7. Effect of Proanthocyanidin on Rumen Microbes and Ammonia Formation
8. Effect of Proanthocyanidin on Intestinal Amino Acid Absorption
9. Effect of Proanthocyanidin on Intestinal Parasites
10. Effect of Proanthocyanidin on Pasture Bloat
11. Effect of Proanthocyanidin on Enteric Methane Emissions
12. Absorption of Proanthocyandin and Health Benefits
13. Effect of Proanthocyanidin on Animal Performance and Animal Product Quality
14. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jonker, A.; Yu, P. The role of proanthocyanidins complex in structure and nutrition interaction in alfalfa forage. Int. J. Mol. Sci. 2016, 17, 793. [Google Scholar] [CrossRef] [PubMed]
- Marles, M.A.S.; Ray, H.; Gruber, M.Y. New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 2003, 64, 367–383. [Google Scholar] [CrossRef]
- Schwinn, K.E.; Davies, K.M. Flavonoids. In Plant Pigments and Their Manipulation; Davies, K.M., Ed.; Annual Plant Revieuws; Blackwell Publishing: Oxford, UK, 2009; Volume 14, Chapter 4; pp. 92–149. [Google Scholar]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin biosynthesis in plants: Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 2003, 278, 31647–31656. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-Y.; Sharma, S.B.; Dixon, R.A. Anthocyanidin reductases from Medicago truncatula and Arabidopsis thaliana. Arch. Biochem. Biophys. 2004, 422, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Theodoridou, K.; Aufrère, J.; Niderkorn, V.; Andueza, D.; Le Morvan, A.; Picard, F.; Baumont, R. In vitro study of the effects of condensed tannins in sainfoin on the digestive process in the rumen at two vegetation cycles. Anim. Feed Sci. Technol. 2011, 170, 147–159. [Google Scholar] [CrossRef]
- Makkar, H.P.; Mueller-Harvey, I.; Hagerman, A.E. Quantification of Tannins in Tree Foilage: A Laboratory Manual for the FAO/IAEA; FAO: Vienna, Austria, 2000. [Google Scholar]
- Waghorn, G.C.; Shelton, I.D. Effect of condensed tannins in Lotus corniculatus on the nutritive value of pasture for sheep. J. Agric. Sci. 1997, 128, 365–372. [Google Scholar] [CrossRef]
- Kleindt, C.K.; Stracke, R.; Mehrtens, F.; Weisshaar, B. Expression analysis of flavonoid biosynthesis genes during Arabidopsis thaliana silique and seed development with a primary focus on the proanthocyanidin biosynthetic pathway. BMC Res. Notes 2010, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pang, Y.; Dixon, R.A. The mysteries of proanthocyanidin transport and polymerization. Plant Physiol. 2010, 153, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Iwaasa, A.D.; Wang, Y.; Jin, L.; Han, G.; Zhao, M. Condensed tannins concentration of selected prairie legume forages as affected by phenological stages during two consecutive growth seasons in western Canada. Can. J. Plant Sci. 2014, 94, 817–826. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; McCallum, J.; Muir, A.D.; Lees, G.L.; Bohm, B.A.; Towers, G.H.N.; Gruber, M.Y. Purification and characterization of a proanthocyanidin polymer from seed of alfalfa (Medicago sativa cv. Beaver). J. Agric. Food Chem. 1993, 41, 565–569. [Google Scholar] [CrossRef]
- Fraser, K.; Collette, V.; Hancock, K.R. Characterization of proanthocyanidins from seeds of perennial ryegrass (Lolium perenne L.) and tall fescue (Festuca arundinacea) by liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2016, 64, 6676–6684. [Google Scholar] [CrossRef] [PubMed]
- Zeller, W.E.; Sullivan, M.L.; Mueller-Harvey, I.; Grabber, J.H.; Ramsay, A.; Drake, C.; Brown, R.H. Protein precipitation behavior of condensed tannins from Lotus pedunculatus and Trifolium repens with different mean degrees of polymerization. J. Agric. Food Chem. 2015, 63, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Meagher, L.P.; Foo, L.Y.; Lane, G.A.; Fraser, K.; Rumball, W. Floral procyanidins of the forage legume red clover (Trifolium pratense L.). J. Agric. Food Chem. 2004, 52, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- McNabb, W.C.; Peters, J.S.; Foo, L.Y.; Waghorn, G.C.; Jackson, F.S. Effect of condensed tannins prepared from several forages on the in vitro precipitation of ribulose-1,5-bis-phosphate carboxylase (Rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1). J. Sci. Food Agric. 1998, 77, 201–212. [Google Scholar] [CrossRef]
- Jackson, F.S.; McNabb, W.C.; Barry, T.N.; Foo, Y.L.; Peters, J.S. The condensed tannin content of a range of subtropical and temperate forages and the reactivity of condensed tannin with ribulose-1,5-bis-phosphate carboxylase (Rubisco) protein. J. Sci. Food Agric. 1996, 72, 483–492. [Google Scholar] [CrossRef]
- Lees, G.L.; Suttill, N.H.; Gruber, M.Y. Condensed tannins in sainfoin. 1. A histological and cytological survey of plant tissue. Can. J. Bot. 1993, 71, 1147–1152. [Google Scholar] [CrossRef]
- Theodoridou, K.; Aufrère, J.; Andueza, D.; Pourrat, J.; Le Morvan, A.; Stringano, E.; Mueller-Harvey, I.; Baumont, R. Effects of condensed tannins in fresh sainfoin (Onobrychis viciifolia) on in vivo and in situ digestion in sheep. Anim. Feed Sci. Technol. 2010, 160, 23–38. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; McCallum, J.; Muir, A.D.; Bohm, B.A.; Towers, G.H.N.; Gruber, M.Y. Developmental changes in the composition of proanthocyanidins from leaves of sainfoin (Onobrychis viciifolia Scop.) as determined by HPLC analysis. J. Agric. Food Chem. 1993, 41, 1066–1070. [Google Scholar] [CrossRef]
- Lees, G.L.; Gruber, M.Y.; Suttill, N.H. Condensed tannins in sainfoin. II. Occurrence and changes during leaf development. Can. J. Bot. 1995, 73, 1540–1547. [Google Scholar] [CrossRef]
- Azuhnwi, B.N.; Boller, B.; Martens, M.; Dohme-Meier, F.; Ampuero, S.; Günter, S.; Kreuzer, M.; Hess, H.D. Morphology, tannin concentration and forage value of 15 Swiss accessions of sainfoin (Onobrychis viciifolia Scop.) as influenced by harvest time and cultivation site. Grass Forage Sci. 2011, 66, 474–487. [Google Scholar] [CrossRef]
- Azuhnwi, B.N.; Boller, B.; Dohme-Meier, F.; Hess, H.D.; Kreuzer, M.; Stringano, E.; Mueller-Harvey, I. Exploring variation in proanthocyanidin composition and content of sainfoin (Onobrychis viciifolia). J. Sci. Food Agric. 2013, 93, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Stienezen, M.; Waghorn, G.C.; Douglas, G.B. Digestibility and effects of condensed tannins on digestion of sulla (Hedysarum coronarium) when fed to sheep. N. Z. J. Agric. Res. 1996, 39, 215–221. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Xu, Z.; Schellenberg, M.P.; Zhang, Y.G.; Liu, X.L.; McAllister, T.A. Effect of condensed tannins on ruminal degradability of purple prairie clover (Dalea purpurea Vent.) harvested at two growth stages. Anim. Feed Sci. Technol. 2012, 176, 17–25. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; McAllister, T.A.; Ominski, K.H. Condensed tannin concentrations found in vegetative and mature forage legumes grown in western Canada. Can. J. Plant Sci. 2011, 91, 669–675. [Google Scholar] [CrossRef]
- Lees, G.L. Effect of high temperature on condensed tannin accumulation in leaf tissues of big trefoil (Lotus uliginosus Schkuhr). J. Sci. Food Agric. 1994, 65, 415–421. [Google Scholar] [CrossRef]
- Barry, T.N.; Duncan, S.J. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep: 1. Voluntary intake. Br. J. Nutr. 1984, 51, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.N.; Forss, D.A. The condensed tannin content of vegetative Lotus pedunculatus, its regulation by fertiliser application, and effect upon protein solubility. J. Sci. Food Agric. 1983, 34, 1047–1056. [Google Scholar] [CrossRef]
- Tiemann, T.T.; Franco, L.H.; Ramírez, G.; Kreuzer, M.; Lascano, C.E.; Hess, H.D. Influence of cultivation site and fertilisation on the properties of condensed tannins and in vitro ruminal nutrient degradation of Calliandra calothyrsus, Flemingia macrophylla and Leucaena leucocephala. Anim. Feed Sci. Technol. 2010, 157, 30–40. [Google Scholar] [CrossRef]
- Tiemann, T.T.; Cortés, J.E.; Pabón, M.L.; Hess, H.D.; Kreuzer, M.; Carulla, J.E. In vitro evidence for the importance of cultivation conditions on the effects of Calliandra tannins on ruminal escape of soybean protein and its post-ruminal degradability. J. Anim. Physiol. Anim. Nutr. 2010, 94, e225–e230. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.M.; Tanner, C.E.; Rothen, M.; Bullington, J. Wound-induced accumulations of condensed tannins in turtlegrass, Thalassia testudinum. Aquat. Bot. 2008, 89, 27–33. [Google Scholar] [CrossRef]
- Lees, G.L.; Howarth, R.E.; Goplen, B.P. Morphological characteristics of leaves from some legume forages: Relation to digestion and mechanical strength. Can. J. Bot. 1982, 60, 2126–2132. [Google Scholar] [CrossRef]
- Cheng, K.J.; Fay, J.P.; Howarth, R.E.; Costerton, J.W. Sequence of events in the digestion of fresh legume leaves by rumen bacteria. Appl. Environ. Microbiol. 1980, 40, 613–625. [Google Scholar] [PubMed]
- McAllister, T.A.; Bae, H.D.; Jones, G.A.; Cheng, K.J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [PubMed]
- Tanner, G.J.; Moore, A.E.; Larkin, P.J. Proanthocyanidins inhibit hydrolysis of leaf proteins by rumen microflora in vitro. Br. J. Nutr. 1994, 71, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; McNabb, W.C.; Barry, T.N.; Peters, J.S. Solubilization and degradation of ribulose-1,5-bis-phosphate carboxylase/oxygenase (EC 4.1.1.39; Rubisco) protein from white clover (Trifolium repens) and Lotus corniculatus by rumen microorganisms and the effect of condensed tannins on these processes. J. Agric. Sci. 2000, 134, 305–317. [Google Scholar] [CrossRef]
- Margan, J.L.; Vetter, R.L.; Jordan, D.J.; Wright, P.C. The effect of the condensed tannins of sainfoin (Onobrychis viciaefolia) on the release of soluble leaf protein into the food bolus of cattle. Proc. Nutr. Soc. 1976, 35, 95A–97A. [Google Scholar]
- Aufrère, J.; Dudilieu, M.; Poncet, C.; Baumont, R. Effect of Condensed Tannins in Sainfoin on in vitro Protein Solubility of Lucerne. In Proceedings of the International Grassland Congress, Dublin, Ireland, 26 June–1 July 2005; Wageningen Academic Publisher: Wageningen, The Netherlands; Dublin, Ireland, 2005; p. 248. [Google Scholar]
- Aufrère, J.; Theodoridou, K.; Mueller-Harvey, I.; Yu, P.; Andueza, D. Ruminal dry matter and nitrogen degradation in relation to condensed tannin and protein molecular structures in sainfoin (Onobrychis viciifolia) and lucerne (Medicago sativa). J. Agric. Sci. 2014, 152, 333–345. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Shelton, I.D.; McNabb, W.C.; McCutcheon, S.N. Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrougenous aspects. J. Agric. Sci. 1994, 123, 109–119. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Ulyatt, M.J.; John, A.; Fisher, M.T. The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus. Br. J. Nutr. 1987, 57, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Hutchinson, K.J.; Revell, D.K.; Brookes, I.M.; McNabb, W.C. The effect of condensed tannins in sainfoin (Onobrychis viciifolia) and sulla (Hedysarum coronarium) on the digestion of amino acids in sheep. Proc. N. Z. Soc. Anim. Prod. 2001, 61, 116–119. [Google Scholar]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol-protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wei, C.-I.; Johnson, M.G. Are tannins a double-edged sword in biology and health? Trends Food Sci. Technol. 1998, 9, 168–175. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Bate-Smith, E.C. Haemanalysis of tannins: The concept of relative astringency. Phytochemistry 1973, 12, 907–912. [Google Scholar] [CrossRef]
- Jones, W.T.; Broadhurst, R.B.; Lyttleton, J.W. The condensed tannins of pasture legume species. Phytochemistry 1976, 15, 1407–1409. [Google Scholar] [CrossRef]
- Horigome, T.; Kumar, R.; Okamoto, K. Effects of condensed tannins prepared from leaves of fodder plants on digestive enzymes in vitro and in the intestine of rats. Br. J. Nutr. 1988, 60, 275–285. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Martinez, T.; Bae, H.D.; Muir, A.D.; Yanke, L.J.; Jones, G.A. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation and inhibitory effects of cellulose digestion. J. Chem. Ecol. 2005, 31, 20–2049. [Google Scholar] [CrossRef] [PubMed]
- Naumann, H.D.; Hagerman, A.E.; Lambert, B.D.; Muir, J.P.; Tedeschi, L.O.; Kothmann, M.M. Molecular weight and protein-precipitating ability of condensed tannins from warm-season perennial legumes. J. Plant Interact. 2014, 9, 212–219. [Google Scholar] [CrossRef]
- Huang, X.D.; Liang, J.B.; Tan, H.Y.; Yahya, R.; Khamseekhiew, B.; Ho, Y.W. Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Anim. Feed Sci. Technol. 2010, 159, 81–87. [Google Scholar] [CrossRef]
- Aerts, R.J.; McNabb, W.C.; Molan, A.; Brand, A.; Barry, T.N.; Peters, J.S. Condensed tannins from Lotus corniculatus and Lotus pedunculatus exert different effects on the in vitro rumen degradation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) protein. J. Sci. Food Agric. 1999, 79, 79–85. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Lachmann, P.; Ramsay, A.; Green, R.J.; Mueller-Harvey, I. Identification of structural features of condensed tannins that affect protein aggregation. PLoS ONE 2017, 12, e0170768. [Google Scholar] [CrossRef] [PubMed]
- Saminathan, M.; Tan, H.; Sieo, C.; Abdullah, N.; Wong, C.; Abdulmalek, E.; Ho, Y. Polymerization degrees, molecular weights and protein-binding affinities of condensed tannin fractions from a Leucaena leucocephala hybrid. Molecules 2014, 19, 7990–8010. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Waffo-Teguo, P.; Jourdes, M.; Li, H.; Teissedre, P.-L. Chemical affinity between tannin size and salivary protein binding abilities: Implications for wine astringency. PLoS ONE 2016, 11, e0161095. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.T.; McNeill, D.M. Characterisation of Leucaena condensed tannins by size and protein precipitation capacity. J. Sci. Food Agric. 2001, 81, 1113–1119. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kilmister, R.L.; Kelm, M.A.; Downey, M.O. Impact of condensed tannin size as individual and mixed polymers on bovine serum albumin precipitation. Food Chem. 2014, 160, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Foo, L.Y.; McNabb, W.C. The effect of different molecular weight procyanidins on in vitro protein degradation. Asian Australas. J. Anim. Sci. 2000, 13, 43–46. [Google Scholar]
- Guimarães-Beelen, P.M.; Berchielli, T.T.; Beelen, R.; Medeiros, A.N. Influence of condensed tannins from Brazilian semi-arid legumes on ruminal degradability, microbial colonization and ruminal enzymatic activity in Saanen goats. Small Rumin. Res. 2006, 61, 35–44. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Rumball, W.; Lane, G.A.; Fraser, K.; Foo, L.Y.; Yu, M.; Meagher, L.P. Variation of proanthocyanidins in Lotus species. J. Chem. Ecol. 2006, 32, 1797–1816. [Google Scholar] [CrossRef] [PubMed]
- Meagher, L.P.; Lane, G.; Sivakumaran, S.; Tavendale, M.H.; Fraser, K. Characterization of condensed tannins from Lotus species by thiolytic degradation and electrospray mass spectrometry. Anim. Feed Sci. Technol. 2004, 117, 151–163. [Google Scholar] [CrossRef]
- Marais, J.P.J.; Mueller-Harvey, I.; Brandt, E.V.; Ferreira, D. Polyphenols, condensed tannins, and other natural products in Onobrychis viciifolia (sainfoin). J. Agric. Food Chem. 2000, 48, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- Tibe, O.; Meagher, L.P.; Fraser, K.; Harding, D.R.K. Condensed tannins and flavonoids from the forage legume sulla (Hedysarum coronarium). J. Agric. Food Chem. 2011, 59, 9402–9409. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Molan, A.L.; Meagher, L.P.; Kolb, B.; Foo, L.Y.; Lane, G.A.; Attwood, G.A.; Fraser, K.; Tavendale, M. Variation in antimicrobial action of proanthocyanidins from Dorycnium rectum against rumen bacteria. Phytochemistry 2004, 65, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.M.; Alkhafadji, L.; Stringano, E.; Nilsson, S.; Mueller-Harvey, I.; Udén, P. Relationship between condensed tannin structures and their ability to precipitate feed proteins in the rumen. J. Sci. Food Agric. 2014, 94, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Hao, Y.-Q.; Jin, L.; Xu, Z.-J.; McAllister, T.; Wang, Y. Anti-escherichia coli O157:H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Soliva, C.R.; Wyss, U.; Kreuzer, M.; Dohme, F. Palatability in sheep and in vitro nutritional value of dried and ensiled sainfoin (Onobrychis viciifolia) birdsfoot trefoil (Lotus corniculatus), and chicory (Cichorium intybus). Arch. Anim. Nutr. 2007, 61, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, V.; Waghorn, G.C.; Woodward, S.; Thom, E. Effects of condensed tannins in white clover flowers on their digestion in vitro. Anim. Feed Sci. Technol. 2008, 142, 44–58. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabbb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; Muir, A.D.; Bohm, B.A.; Towers, G.H.N.; Gruber, M.Y. The proanthocyanidin polymers in some species of Onobrychis. Phytochemistry 1993, 34, 113–117. [Google Scholar] [CrossRef]
- Foo, L.Y.; Newman, R.; Waghorn, G.; McNabb, W.C.; Ulyatt, M.J. Proanthocyanidins from Lotus corniculatus. Phytochemistry 1996, 41, 617–624. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; McNabb, W.C.; Waghorn, G.; Ulyatt, M.J. Proanthocyanidins from Lotus pedunculatus. Phytochemistry 1997, 45, 1689–1696. [Google Scholar] [CrossRef]
- Yu, P.; Jonker, A.; Gruber, M. Molecular basis of protein structure in proanthocyanidin and anthocyanin-enhanced Lc-transgenic alfalfa in relation to nutritive value using synchrotron-radiation FTIR microspectroscopy: A novel approach. Spectrochim. Acta Mol. 2009, 73, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yari, M.; Valizadeh, R.; Naserian, A.A.; Jonker, A.; Yu, P. Protein molecular structures in alfalfa hay cut at three stages of maturity and in the afternoon and morning and relationship with nutrient availability in ruminants. J. Sci. Food Agric. 2013, 93, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; Attwood, G.T.; Reilly, K.; Sun, W.; Peters, J.S.; Barry, T.N.; McNabb, W.C. Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep. Can. J. Mcrobiol. 2002, 48, 911–921. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.N.; Manley, T.R. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep 2. Quantitative digestion of carbohydrates and proteins. Br. J. Nutr. 1984, 51, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.R.; Ehlke, N.J. Condensed tannins in birdsfoot trefoil: Genetic relationships with forage yield and quality in NC-83 germplasm. Euphytica 1995, 92, 383–391. [Google Scholar] [CrossRef]
- Grabber, J.H. Forage management effects on protein and fiber fractions, protein degradability, and dry matter yield of red clover conserved as silage. Anim. Feed Sci. Technol. 2009, 154, 284–291. [Google Scholar] [CrossRef]
- Grabber, J.H. Protein fractions in forage legumes containing protein-binding polyphenols: Freeze-drying vs. conservation as hay or silage. Anim. Feed Sci. Technol. 2009, 151, 324–329. [Google Scholar] [CrossRef]
- Terrill, T.; Douglas, G.; Foote, A.; Purchas, R.; Wilson, G.; Barry, T. Effect of condensed tannins upon body growth, wool growth and rumen metabolism in sheep grazing sulla (Hedysarum coronarium) and perennial pasture. J. Agric. Sci. 1992, 119, 265–273. [Google Scholar] [CrossRef]
- Min, B.R.; McNabb, W.C.; Kemp, P.D.; Barry, T.N. Effect of condensed tannins on the production of wool and on its processing characteristics in sheep grazing Lotus corniculatus. Aust. J. Agric. Res. 1998, 49, 597–606. [Google Scholar] [CrossRef]
- Barry, T.N.; Manley, T.R.; Duncan, S.J. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep 4. Sites of carbohydrate and protein digestion as influenced by dietary reactive tannin concentration. Br. J. Nutr. 1986, 55, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G.D.; John, A.; Jones, W.T.; Shelton, I.D. Nutritive value of Lotus corniculatus L. containing low and medium concentrations of condensed tannins for sheep. Proc. N. Z. Soc. Anim. Prod. 1987, 47, 25–30. [Google Scholar]
- Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.D.; Cheng, K.J. Effects of sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [PubMed]
- Min, B.R.; Attwood, G.T.; McNabb, W.C.; Molan, A.L.; Barry, T.N. The effect of condensed tannins from Lotus corniculatus on the proteolytic activities and growth of rumen bacteria. Anim. Feed Sci. Technol. 2005, 121, 45–58. [Google Scholar] [CrossRef]
- Smith, A.H.; Mackie, R.I. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Yanez Ruiz, D.R.; Moumen, A.; Martin Garcia, A.I.; Molina Alcaide, E. Ruminal fermentation and degradation patterns, protozoa population, and urinary purine derivatives excretion in goats and wethers fed diets based on two-stage olive cake: Effect of PEG supply. J. Anim. Sci. 2004, 82, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Vaithiyanathan, S.; Bhatta, R.; Mishra, A.S.; Prasad, R.; Verma, D.L.; Singh, N.P. Effect of feeding graded levels of Prosopis cineraria leaves on rumen ciliate protozoa, nitrogen balance and microbial protein supply in lambs and kids. Anim. Feed Sci. Technol. 2007, 133, 177–191. [Google Scholar] [CrossRef]
- Veira, D.M. The role of ciliate protozoa in nutrition of the ruminant. J. Anim. Sci. 1986, 63, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.P. Effect of rumen protozoa on nitrogen utilization by ruminants. J. Nutr. 1996, 126, 1335S–1346S. [Google Scholar] [PubMed]
- Doranalli, K.; Mutsvangwa, T. Feeding sunflower oil to partially defaunate the rumen increases nitrogen retention, urea-nitrogen recycling to the gastrointestinal tract and the anabolic use of recycled urea-nitrogen in growing lambs. Br. J. Nutr. 2011, 105, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G.C. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Woodward, S.L.; Waghorn, G.C.; Watkins, K.A.; Bryant, M.A. Feeding birdsfoot trefoil (Lotus corniculatus) reduces the environmental impacts of dairy farming. Proc. N. Z. Soc. Anim. Prod. 2009, 69, 179–183. [Google Scholar]
- Hoekstra, N.J.; Schulte, R.P.O.; Struik, P.C.; Lantinga, E.A. Pathways to improving the N efficiency of grazing bovines. Eur. J. Agron. 2007, 26, 363–374. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia) with fraction 1 leaf protein and with submaxillary mucoprotein and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [PubMed]
- Boerner, B.J.; Byers, F.M.; Schelling, G.T.; Coppock, C.E.; Greene, L.W. Trona and sodium bicarbonate in beef cattle diets: Effects on pH and volatile fatty acid concentrations. J. Anim. Sci. 1987, 65, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, W.E.; Noller, C.H. Gastrointestinal tract pH and starch in feces of ruminants. J. Anim. Sci. 1977, 44, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, S.C.L.; Muller, K.E.; Kuiper, R.; Noordhuizen, J.P.T.M. Studies on the pH Value of Abomasal Contents in Dairy Cows during the First 3 Weeks after Calving. J. Vet. Med. A 2002, 49, 157–160. [Google Scholar] [CrossRef]
- Aufrère, J.; Dudilieu, M.; Andueza, D.; Poncet, C.; Baumont, R. Mixing sainfoin and lucerne to improve the feed value of legumes fed to sheep by the effect of condensed tannins. Animal 2013, 7, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, I.W.; Norton, B.W. The digestion of dietary protein bound by condensed tannins in the gastro-intestinal tract of sheep. Anim. Feed Sci. Technol. 2008, 142, 197–209. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Waghorn, G.C.; McNabb, W.C. Condensed tannins and gastro-intestinal parasites in sheep. Proc. N. Z. Grassl. Assoc. 1999, 61, 57–61. [Google Scholar]
- Niezen, J.H.; Charleston, W.A.G.; Robertson, H.A.; Shelton, D.; Waghorn, G.C.; Green, R. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and development of immunity to gastrointestinal nematodes. Vet. Parasitol. 2002, 105, 229–245. [Google Scholar] [CrossRef]
- Niezen, J.H.; Robertson, H.A.; Waghorn, G.C.; Charleston, W.A.G. Production, faecal egg counts and worm burdens of ewe lambs which grazed six contrasting forages. Vet. Parasitol. 1998, 80, 15–27. [Google Scholar] [CrossRef]
- Robertson, H.A.; Niezen, J.H.; Waghorn, G.C.; Charleston, W.A.G.; Jinlong, M. The effect of six herbages on liveweight gain, wool growth and faecal egg count of parasitised ewe lambs. Proc. N. Z. Soc. Anim. Prod. 1995, 55, 199–201. [Google Scholar]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Quijada, J.; Fryganas, C.; Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I.; Hoste, H. Anthelmintic activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. J. Agric. Food Chem. 2015, 63, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Desrues, O.; Fryganas, C.; Ropiak, H.M.; Mueller-Harvey, I.; Enemark, H.L.; Thamsborg, S.M. Impact of chemical structure of flavanol monomers and condensed tannins on in vitro anthelmintic activity against bovine nematodes. Parasitology 2016, 143, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G.C.; Molan, A.L. Effect of condensed tannins in Dorycnium rectum on its nutritive value and on the development of sheep parasite larvae. Proc. N. Z. Soc. Anim. Prod. 2001, 63, 273–277. [Google Scholar]
- Waghorn, T.S.; Molan, A.L.; Deighton, M.; Alexander, R.A.; Leathwick, D.M.; McNabb, W.C.; Meagher, L.P. In vivo anthelmintic activity of Dorycnium rectum and grape seed extract against Ostertagia (Teladorsagia) circumcincta and Trichostrongylus colubriformis in sheep. N. Z. Vet. J. 2006, 54, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lees, G.L. Condensed tannis in some forage legumes. Basic Life Sci. 1992, 59, 915–934. [Google Scholar] [PubMed]
- Coulman, B.; Goplen, B.; Majak, W.; McAllister, T.A.; Cheng, K.J.; Berg, B.; Hall, J.W.; McCartney, D.; Acharya, S.N. A review of the development of a bloat-reduced alfalfa cultivar. Can. J. Plant Sci. 2000, 80, 487–491. [Google Scholar] [CrossRef]
- Tanner, G.J.; Moate, P.J.; Davis, L.A.; Laby, R.H.; Yuguang, L.; Larkin, P.J. Proanthocyanidins (condensed tannin) destabilize plant protein foams in a dose dependent manner. Aust. J. Agric. Res. 1995, 46, 1101–1109. [Google Scholar] [CrossRef]
- Fay, J.P.; Cheng, K.J.; Hanna, M.R.; Howarth, R.E.; Costerton, J.W. In vitro digestion of bloat-safe and bloat-causing legumes by rumen microorganisms: Gas and foam production. J. Dairy Sci. 1980, 63, 1273–1281. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Fulford, J.D.; Puchala, R. Effect of feed additives on in vitro and in vivo rumen characteristics and frothy bloat dynamics in steers grazing wheat pasture. Anim. Feed Sci. Technol. 2005, 123–124, 615–629. [Google Scholar] [CrossRef]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Coulman, B.E.; Wang, Y.; Cheng, K.J. A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can. J. Plant Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef]
- Clarke, R.T.J.; Reid, C.S.W. Foamy bloat of cattle. A review. J. Dairy Sci. 1974, 57, 753–785. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Fina, L.R.; Bartley, E.E.; Anthony, H.D. Endotoxic activity of cell-free rumen fluid from cattle fed hay or grain. Can. J. Microbiol. 1978, 24, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Hume, M.E. In vitro bacterial growth and in vivo ruminal microbiota populations associated with bloat in steers grazing wheat forage. J. Anim. Sci. 2006, 84, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA-HC1 protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Jones, W.T. Bloat in cattle 46. Potential of dock (Rumex obtusifolius) as an antibloat agent for cattle. N. Z. J. Agric. Res. 1989, 32, 227–235. [Google Scholar] [CrossRef]
- Meagher, L.P.; Spencer, P.; Lane, G.; Sivakumaran, S.; Fraser, K. What do green tea, grapes seed, and dock have in common? Chem. N. Z. 2005, 69, 6–9. [Google Scholar]
- Taylor, P.W.; Hamilton-Miller, J.M.T.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2005, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G.C.; Tavendale, M.H.; Woodfield, D.R. Methagonesis from forage fed to sheep. Proc. N. Z. Grassl. Assoc. 2002, 64, 167–171. [Google Scholar]
- Waghorn, G.C.; Clark, D.A. Greenhouse gas mitigation opportunities with immediate application to pastoral grazing for ruminants. Int. Congr. Ser. 2006, 1293, 107–110. [Google Scholar] [CrossRef]
- Pinares-Patiño, C.S.; Ulyatt, M.J.; Waghorn, G.C.; Lassey, K.R.; Barry, T.N.; Holmes, C.W.; Johnson, D.E. Methane emission by alpaca and sheep fed on lucerne hay or grazed on pastures of perennial ryegrass/white clover or birdsfoot trefoil. J. Agric. Sci. 2003, 140, 215–226. [Google Scholar] [CrossRef]
- Woodward, S.L.; Waghhorn, G.C.; Ulyatt, M.J.; Lassey, K.R. Early indications that feeding Lotus will reduce methane emissions from ruminants. Proc. N. Z. Soc. Anim. Prod. 2001, 61, 23–26. [Google Scholar]
- Woodward, S.L.; Waghhorn, G.C.; Lassey, K.R.; Laboyrie, P.G. Does feeding sulla (Hedysarum coronarium) reduce methane emission from dairy cows? Proc. N. Z. Soc. Anim. Prod. 2002, 62, 227–230. [Google Scholar]
- Woodward, S.L.; Waghhorn, G.C.; Laboyrie, P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 160–164. [Google Scholar]
- Puchala, R.; Min, B.R.; Goetsch, A.L.; Sahlu, T. The effect of a condensed tannin-containing forage on methane emission by goats. J. Anim. Sci. 2005, 83, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Hatew, B.; Hayot Carbonero, C.; Stringano, E.; Sales, L.F.; Smith, L.M.J.; Mueller-Harvey, I.; Hendriks, W.H.; Pellikaan, W.F. Diversity of condensed tannin structures affects rumen in vitro methane production in sainfoin (Onobrychis viciifolia) accessions. Grass Forage Sci. 2015, 70, 474–490. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 2005, 123–124, 403–419. [Google Scholar] [CrossRef]
- Bouchard, K.; Wittenberg, K.M.; Legesse, G.; Krause, D.O.; Khafipour, E.; Buckley, K.E.; Ominski, K.H. Comparison of feed intake, body weight gain, enteric methane emission and relative abundance of rumen microbes in steers fed sainfoin and lucerne silages under western Canadian conditions. Grass Forage Sci. 2015, 70, 116–129. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Mc Geough, E.J.; Acharya, S.; McAllister, T.A.; McGinn, S.M.; Harstad, O.M.; Beauchemin, K.A. Enteric methane emission, diet digestibility, and nitrogen excretion from beef heifers fed sainfoin or alfalfa. J. Anim. Sci. 2013, 91, 4861–4874. [Google Scholar] [CrossRef] [PubMed]
- Iwaasa, A.; Lemke, R.L.; Brikedal, E. Comparing alfalfa-grass versus Sainfoin pastures in beef and forage production and methane emissions. In Proceedings of the Joint Annual Meetings of the American Forage and Grassland Council and the Society for Range Management, Louisville, KY, USA, 26–31 January 2008. [Google Scholar]
- Iwaasa, A. Strategies to reduce greenhouse gas emissions through feeding and grazing management. In Proceedings of the 19th Annual Conference of the Saskatchewan Soil Conservation Association, Saskatoon, SK, Canada, 12–13 February 2007; pp. 97–104. [Google Scholar]
- Huyen, N.T.; Desrues, O.; Alferink, S.J.J.; Zandstra, T.; Verstegen, M.W.A.; Hendriks, W.H.; Pellikaan, W.F. Inclusion of sainfoin (Onobrychis viciifolia) silage in dairy cow rations affects nutrient digestibility, nitrogen utilization, energy balance, and methane emissions. J. Dairy Sci. 2016, 99, 3566–3577. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Pellikaan, W.F.; Hendriks, W.H.; Uwimana, G.; Bongers, L.J.G.M.; Becker, P.M.; Cone, J.W. A novel method to determine simultaneously methane production during in vitro gas production using fully automated equipment. Anim. Feed Sci. Technol. 2011, 168, 196–205. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.-P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Waghorn, G.C.; Woolley, D.J.; McNabb, W.C.; Barry, T.N. Assay and digestion of C-labelled condensed tannins in the gastrointestinal tract of sheep. Br. J. Nutr. 1994, 72, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Auger, C.; Mullen, W.; Bornet, A.; Rouanet, J.-M.; Crozier, A.; Teissedre, P.-L. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br. J. Nutr. 2005, 94, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Stoupi, S.; Williamson, G.; Viton, F.; Barron, D.; King, L.J.; Brown, J.E.; Clifford, M.N. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C] Procyanidin B2 in male rats. Drug Metab. Dispos. 2010, 38, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Akiyama, H.; Kanda, T.; Ohtake, Y.; Goda, Y. Apple procyanidin oligomers absorption in rats after oral administration: Analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Wein, S.; Beyer, B.; Gohlke, A.; Blank, R.; Metges, C.C.; Wolffram, S. Systemic absorption of catechins after intraruminal or intraduodenal application of a green tea extract in cows. PLoS ONE 2016, 11, e0159428. [Google Scholar] [CrossRef] [PubMed]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; di Grigoli, A.; Claps, S. Effects of sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Q.; Jin, L.; Xu, Z.; Barbieri, L.R.; Acharya, S.; Hu, T.M.; McAllister, T.A.; Stanford, K.; Wang, Y. Effects of purple prairie clover (Dalea purpurea Vent.) on feed intake, nutrient digestibility and faecal shedding of Escherichia coli O157:H7 in lambs. Anim. Feed Sci. Technol. 2015, 207, 51–61. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Tam, N.F.-Y.; Lin, Y.-M.; Ding, Z.-H.; Chai, W.-M.; Wei, S.-D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-D.; Zhou, H.-C.; Lin, Y.-M. Antioxidant activities of fractions of polymeric procyanidins from stem bark of Acacia confusa. Int. J. Mol. Sci. 2011, 12, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Ulyatt, M.J. The feeding value of herbage: Can it be improved? N. Z. Agric. Sci. 1981, 15, 200–205. [Google Scholar]
- Waghorn, G.C.; Burke, J.L.; Kolver, E.S. Principles of feeding value. In Pasture and Supplements for Grazing Animals; Rattary, P.V., Brookes, I.M., Nicol, A.M., Eds.; New Zealand Society of Animal Production: Hamilton, New Zealand, 2007; pp. 35–59. [Google Scholar]
- Aerts, R.J.; Barry, T.N.; McNabb, W.C. Polyphenols and agriculture: Beneficial effects of proanthocyanidins in forages. Agric. Ecosyst. Environ. 1999, 75, 1–12. [Google Scholar] [CrossRef]
- Hartnell, G.F.; Satter, L.D. Determination of rumen fill, retention time and ruminal turnover rates of ingesta at different stages of lactation in dairy cows. J. Anim. Sci. 1979, 48, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.J.; Moss, B.R. Nutritional value of sainfoin hay compared with alfalfa hay. J. Dairy Sci. 1981, 64, 206–210. [Google Scholar] [CrossRef]
- Khalilvandi-Behroozyar, H.; Dehghan-Banadaky, M.; Rezayazdi, K. Palatability, in situ and in vitro nutritive value of dried sainfoin (Onobrychis viciifolia). J. Agric. Sci. 2010, 148, 723–733. [Google Scholar] [CrossRef]
- Niezen, J.H.; Waghorn, T.S.; Raufaut, K.; Robertson, H.A.; McFarlane, R.G. Lamb weight gain and faecal egg count when grazing one of seven herbages and dosed with larvae for six weeks. Proc. N. Z. Soc. Anim. Prod. 1994, 54, 15–18. [Google Scholar]
- Jeronimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Sales-Baptista, E.; Lopes, O.; Silva, F.C. Tannins in ruminant nutrition: Impact on animal performance and quality of edible products. In Tannins: Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2016; pp. 121–168. [Google Scholar]
- Morales, R.; Ungerfeld, E.M. Use of tannins to improve fatty acids profile of meat and milk quality in ruminants: A review. Chil. J. Agric. Res. 2015, 75, 239–248. [Google Scholar] [CrossRef]
- Jayanegara, A. Ruminal biohydrogenation pattern of poly-unsaturated fatty acid as influenced by dietary tannin. Wartazoa 2013, 23, 8–14. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Lane, G.A.; Tavendale, M.H.; Barry, T.N.; McNabb, W.C. Pastoral flavour in meat products from ruminants fed fresh forages and its amelioration by forage condensed tannins. Anim. Feed Sci. Technol. 2008, 146, 193–221. [Google Scholar] [CrossRef]
- Elder, R.O.; Keen, J.E.; Siragusa, G.R.; Barkocy-Gallagher, G.A.; Koohmaraie, M.; Laegreid, W.W. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 2000, 97, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian Australas. J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Bach, S.J.; Wang, Y.; McAllister, T.A. Effect of feeding sun-dried seaweed (Ascophyllum nodosum) on fecal shedding of Escherichia coli O157:H7 by feedlot cattle and on growth performance of lambs. Anim. Feed Sci. Technol. 2008, 142, 17–32. [Google Scholar] [CrossRef]
- Berard, N.C.; Holley, R.A.; McAllister, T.A.; Ominski, K.H.; Wittenberg, K.M.; Bouchard, K.S.; Bouchard, J.J.; Krause, D.O. Potential to reduce Escherichia coli shedding in cattle feces by using sainfoin (Onobrychis viciifolia) forage, tested in vitro and in vivo. Appl. Environ. Microbiol. 2009, 75, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, Y.; Iwaasa, A.A.; Li, Y.; Xu, Z.; Schellenberg, M.; Liu, X.L.; McAllister, T.; Stanford, K. Purple prairie clover (Dalea purpurea Vent) reduces fecal shedding of Escherichia coli in pastured cattle. J. Food Prot. 2015, 78, 1434–1441. [Google Scholar] [CrossRef] [PubMed]

| Trait | Legume Species | ||||||
|---|---|---|---|---|---|---|---|
| Sainfoin | Birdsfoot Trefoil | Big Trefoil | Sulla | Alfalfa | White Clover | Red Clover | |
| Forage | |||||||
| Proanthocyanidin (g/kg DM) 1 | |||||||
| Extractable | 44 | 7–36 | 61 | 35–84 | 0 | ND | 0.4 |
| Protein-bound | 38 | 9–13 | 14 | 9–31 | 0.5 | ND | 0.6 |
| Fibre-bound | 5 | 2–3 | 1 | 2–20 | 0 | ND | 0.7 |
| Total | 87 | 21–47 | 77 | 55–84 | 0.5 | 6–12 | 1.7 |
| Forage | Seed | Flower | |||||
| MW (DA) 2 | 2.0–5.1 | 1.8–4.4 | 2.2–3.9 | - | 3.6 | - | - |
| mDP 3 | 4–12 | 6–14 | 8–44 | 3–46 | 5–7 | 10 | 9 |
| Main polymer 3 | Pdelph | Pcyanid | Pdelph | Pdelph | Pcyanid | Pdelph | Pcyanid |
| PD (%) 3 | 36–93 | 40–66 | 80–84 | 73–89 | - | - | - |
| Cis (%) 3 | 47–88 | 84–85 | 76–88 | 69–84 | - | - | - |
| Extender unit (%) 3 | |||||||
| Catechin | 0 | 3–4 | 2–4 | 1–8 | 0 | 0 | 6 |
| Epicatechin | 11–27 | 27–67 | 13–19 | 9–18 | 92 | 0 | 81 |
| Gallocatechin | 7–19 | 5–7 | 6–16 | 14–23 | 0 | 39 | 6 |
| Epigallocatechin | 61–74 | 30–62 | 46–72 | 53–75 | 0 | 56 | 7 |
| Terminal unit (%) 3 | |||||||
| Catechin | 8–23 | 61–82 | 46–51 | 24–32 | 92 | 0 | 95 |
| Epicatechin | 22–47 | 16–21 | 13–20 | 0–6 | 0 | 0 | 5 |
| Gallocatechin | 18–40 | 2–17 | 20–16 | 50–66 | 0 | 48 | 0 |
| Epigallocatechin | 14–35 | 2–4 | 10–14 | 7–22 | 0 | 52 | 0 |
| PCC (µg/mg) 4 | |||||||
| Alfalfa Rubisco | 50 | 80 | 72 | ND | 108 | ND | ND |
| Bovine serum albumin | 269 | 436 | 323 | ND | 348 | ND | ND |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonker, A.; Yu, P. The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract. Int. J. Mol. Sci. 2017, 18, 1105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18051105
Jonker A, Yu P. The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract. International Journal of Molecular Sciences. 2017; 18(5):1105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18051105
Chicago/Turabian StyleJonker, Arjan, and Peiqiang Yu. 2017. "The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract" International Journal of Molecular Sciences 18, no. 5: 1105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18051105






