Chlorpromazine Increases the Expression of Polysialic Acid (PolySia) in Human Neuroblastoma Cells and Mouse Prefrontal Cortex
Abstract
:1. Introduction
2. Results
2.1. Effects of CPZ on PolySia Expression Using IMR-32 Human Neuroblastoma Cells
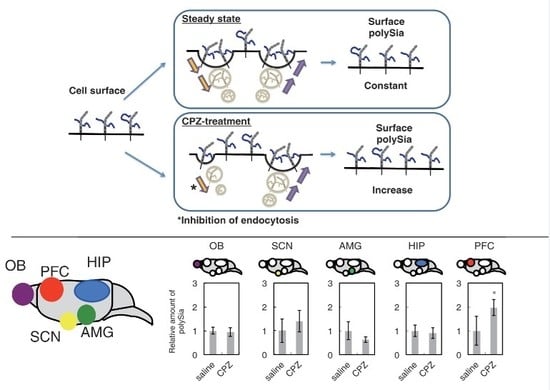
2.2. Effects of CPZ on PolySia Expression Using Mouse Brains
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Treatment of Chlorpromazine (CPZ) Using Neural Cells
4.4. SDS-PAGE and Western Blotting
4.5. Flow Cytometry Analysis
4.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.7. Mild Acid Hydrolysis-Fluorometric Anion-Exchange Chromatography Analysis (MH-FAEC)
4.8. Brefeldin Treatment
4.9. Cell Staining
4.10. Measurement of PolySia on the Cell Surface
4.11. Animals and Ethics Statement
4.12. Drug Treatment
4.13. Preparation of the Brain Sample
4.14. Data Processing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BD | bipolar disorder |
| BDNF | brain-derived neurotrophic factor |
| FGF2 | fibroblast growth factor-2 |
| HIP | hippocampus |
| NCAM | neural cell adhesion molecule |
| SZ | schizophrenia |
References
- Sato, C.; Kitajima, K. Disialic, oligosialic and polysialic acids: Distribution, functions and related disease. J. Biochem. 2013, 154, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Colley, K.J.; Kitajima, K.; Sato, C. Polysialic acid: Biosynthesis, novel functions and applications. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 498–532. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Major, D.; Rutishauser, U. Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J. Biol. Chem. 1994, 269, 23039–23044. [Google Scholar] [PubMed]
- Kanato, Y.; Kitajima, K.; Sato, C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 2008, 18, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Hane, M.; Sumida, M.; Kitajima, K.; Sato, C. Structural and functional impairments of polySia-NCAM synthesized by a mutated polysialyltransferase of a schizophrenic patient. Pure. Appl. Chem. 2012, 84, 1895–1906. [Google Scholar] [CrossRef]
- Hane, M.; Matsuoka, S.; Ono, S.; Miyata, S.; Kitajima, K.; Sato, C. Protective effects of polysialic acid on proteolytic cleavage of FGF2 and proBDNF/BDNF. Glycobiology 2015, 25, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Isomura, R.; Kitajima, K.; Sato, C. Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J. Biol. Chem. 2011, 286, 21535–21545. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Hane, M.; Kitajima, K.; Sato, C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J. Biol. Chem. 2012, 287, 3710–3722. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, D.; Liang, J.; Robitalille, Y.; Quirion, R.; Srivastava, L. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc. Natl. Acad. Sci. USA 1995, 92, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Juan, J.; Varea, E.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Nácher, J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci. Lett. 2012, 530, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yamada, k.; Toyota, T.; Obata, N.; Haga, S.; Yoshida, Y.; Nakamura, N.; Minabe, Y.; Ujike, H.; Sora, I.; et al. Association between polymorphisms in the promoter region of the sialyltransferase 8B (SIAT8B) gene and schizophrenia. Biol. Psychiatry 2006, 59, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Kröcher, T.; Malinovskaja, K.; Jürgenson, M.; Aonurm-Helm, A.; Zharkovskaya, T.; Kalda, A.; Röckle, I.; Sciff, M.; Weinhold, B.; Gerardy-Schahn, R.; et al. Schizophrenia-like phenotype of polysialyltransferase ST8SIA2-deficient mice. Brain Struct. Funct. 2013, 220, 71–83. [Google Scholar]
- Yang, S.Y.; Huh, I.S.; Baek, J.H.; Cho, E.Y.; Choi, M.J.; Ryu, S.; Kim, J.S.; Park, T.; Ha, K.; Hong, K.S. Association between ST8SIA2 and the Risk of Schizophrenia and Bipolar I Disorder across Diagnostic Boundaries. PLoS ONE 2015, 10, e0139413. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Nakayama, J.; Fujimoto, I.; Ikenaka, K.; et al. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res. 2000, 60, 3072–3080. [Google Scholar] [PubMed]
- Hodgkinson, C.; Goldman, D.; Jaeger, J.; Persaud, S.; Kane, J.; Lipsky, R.; Malhotra, A. Disrupted in schizophrenia 1 (DISC1): Association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am. J. Hum. Genet. 2004, 75, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Petursson, H.; Sigurdsson, E.; Steinthorsdottir, V.; Bjornsdottir, S.; Sigmundsson, T; Ghosh, T.; Brynjolfsson, J.; Gunnarsdottir, V.G.; Ivarsson, O.; et al. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002, 71, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, K.; Kolachana, B.; Vakkalanka, R.; Straub, R.; Giegling, I.; Egan, M.; Rujescu, D.; Weinberger, D. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: Influence on risk of schizophrenia. Hum. Genet. 2007, 120, 889–906. [Google Scholar] [CrossRef] [PubMed]
- Strous, R.; Bark, N.; Parsia, S.; Volavka, J.; Lachman, H. Analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia: Evidence for association with aggressive and antisocial behavior. Psychiatry Res. 1997, 69, 71–77. [Google Scholar] [CrossRef]
- Harrison, P.; Weinberger, D. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2005, 10, 40–68. [Google Scholar] [CrossRef] [PubMed]
- Craddock, N.; O’Donovan, M.; Owen, M. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr. Bull. 2006, 32, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.S.; Werge, T.; Sklar, P.; Owen, M.J.; Ophoff, R.A.; O’Donovan, M.C.; Corvin, A.; Cichon, S.; Sullivan, P.F. Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry 2015, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, B.; Moberg, P.; Roalf, D.; Arnold, S.; Gur, R. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch. Gen. Psychiatry 2003, 60, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Cremer, H.; Lange, R.; Christoph, A.; Plomann, M.; Vopper, G.; Roes, J.; Brown, R.; Baldwin, S.; Kraemer, P.; Scheff, S.; et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994, 367, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 2004, 174, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Long, J.M.; Bukalo, O.; Lee, W.; Dityatew, A.; Wynshaw-Boris, A.; Schachner, M; Fukuda, M.; Marth, J.D. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 2004, 279, 32603–32613. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Bukalo, O.; Chazal, G.; Wang, L.; Goridis, C.; Schachner, M.; Gerardy–Schahn, R.; Cremer, H.; Dityatev, A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 2000, 20, 5234–5244. [Google Scholar] [PubMed]
- Weinhold, B.; Seidenfaden, R.; Röckle, I.; Mühlenhoff, M.; Schertzinger, F.; Conzelmann, S.; Marth, J.D.; Gerardy–Schahn, R.; Hildebrandt, H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 2005, 280, 42971–42977. [Google Scholar] [CrossRef] [PubMed]
- Maziade, M.; Roy, M.A.; Chagnon, Y.C.; Cliche, D.; Fournier, J.P.; Montgrain, N.; Dion, C.; Lavallée, J.C.; Garneau, Y.; Gingras, N.; Nicole, L.; et al. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: A dense genome scan in Eastern Quebec families. Mol. Psychiatry 2005, 10, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Sato, C; Kitajima, K. Impact of structural aberrancy of polysialic acid and its synthetic enzyme ST8SIA2 in schizophrenia. Front Cell Neurosci. 2013, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Levinson, D.F. Pharmacologic treatment of schizophrenia. Clin. Ther. 1991, 13, 326–352. [Google Scholar] [PubMed]
- Miyamoto, S.; Miyake, N.; Jarskog, L.F.; Fleischhacker, W.W.; Lieberman, J.A. Pharmacological treatment of schizophrenia: A critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry 2012, 17, 1206–1227. [Google Scholar] [CrossRef] [PubMed]
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Yoshimi, K.; Ren, Y.R.; Seki, T.; Yamada, M.; Ooizumi, H.; Onodera, M.; Saito, Y.; Murayama, S.; Okano, H.; Mizuno, Y.; Mochizuki, H. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann. Neurol. 2005, 58, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Gómez, E.; Gómez-Climent, M.A.; Varea, E.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Martínez-Guijarro, F.J.; Nácher, J. Dopamine acting through D2 receptors modulates the expression of PSA-NCAM, a molecule related to neuronal structural plasticity, in the medial prefrontal cortex of adult rats. Exp. Neurol. 2008, 214, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Cabrini, D.; Sher, E.; Clementi, F. Effects of long-term in vitro exposure to aluminum, cadmium or lead on differentiation and cholinergic receptor expression in a human neuroblastoma cell line. Cell Biol. Toxicol. 1987, 3, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.; Klimke, A; Henning, U. Antipsychotic drugs influence transport of the β-adrenergic antagonist [3H]-dihydroalprenolol into neuronal and blood cells. World J. Biol. Psychiatry 2004, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.; Vimr, E.; Yu, F.; Bassler, B.; Troy, F. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-α-2,8-sialosyl carbohydrate units. J. Biol. Chem. 1987, 262, 3553–3561. [Google Scholar] [PubMed]
- Sato, C.; Inoue, S.; Matsuda, T.; Kitajima, K. Fluorescent-assisted detection of oligosialyl units in glycoconjugates. Anal. Biochem. 1999, 266, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Diestel, S.; Schaefer, D.; Cremer, H.; Schmitz, B. NCAM is ubiquitylated, endocytosed and recycled in neurons. J. Cell Sci. 2007, 120, 4035–4049. [Google Scholar] [CrossRef] [PubMed]
- Miñana, R.; Duran, J.M.; Tomas, M.; Renau-Piqueras, J.; Guerri, C. Neural cell adhesion molecule is endocytosed via a clathrin-dependent pathway. Eur. J. Neurosci. 2001, 13, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.A.; Haugwitz, M.; Lau, D.; Moore, H.P. Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J. Neurosci. 2000, 20, 7334–7344. [Google Scholar] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Mico, J.A.; Lenegre, A.; Steru, M.; Simon, P.; Porsolt, R.D. The automated Tail Suspension Test: A computerized device which differentiates psychotropic drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 1987, 11, 659–671. [Google Scholar] [CrossRef]
- Daniel, J.A.; Chau, N.; Abdel-Hamid, M.K.; Hu, L.; von Kleist, L.; Whiting, A.; Krishnan, S.; Maamary, P.; Joseph, S.R.; Simpson, F.; et al. Phenothiazine-derived antipsychotic drugs inhibit dynamin and clathrin-mediated endocytosis. Traffic 2015, 16, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Subtil, A.; Hémar, A.; Dautry-Varsat, A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J. Cell. Sci. 1994, 107, 3461–3468. [Google Scholar] [CrossRef]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell. Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Hane, M.; Kitajima, K.; Sato, C. Effects of intronic single nucleotide polymorphisms (iSNPs) of a polysialyltransferase, ST8SIA2 gene found in psychiatric disorders on its gene products. Biochem. Biophys. Res. Commun. 2016, 478, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Sumida, M; Hane, M.; Yabe, U.; Shimoda, Y.; Pearce, O.M.; Kiso, M.; Miyagi, T.; Sawada, M.; Varki, A.; Kitajima, K.; Sato, C. Rapid Trimming of Cell Surface Polysialic Acid (PolySia) by Exovesicular Sialidase Triggers Release of Preexisting Surface Neurotrophin. J. Biol. Chem. 2015, 290, 13202–13214. [Google Scholar] [CrossRef] [PubMed]
- Tapper, H.; Sundler, R. Role of lysosomal and cytosolic pH in the regulation of macrophage lysosomal enzyme secretion. Biochem. J. 1990, 272, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Kitajima, K.; Inoue, S.; Inoue, Y. Detection, isolation, and characterization of oligo/poly(sialic acid) and oligo/poly(deaminoneuraminic acid) units in glycoconjugates. Anal. Biochem. 1992, 202, 25–34. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K.; Inoue, S.; Seki, T.; Troy, F.A.; Inoue, Y. Characterization of the antigenic specificity of four different anti-(α 2-->8-linked polysialic acid) antibodies using lipid-conjugated oligo/polysialic acids. J. Biol. Chem. 1995, 270, 18923–18928. [Google Scholar] [PubMed]
- Nagae, M.; Ikeda, A.; Hane, M.; Hanashima, S.; Kitajima, K.; Sato, C.; Yamaguchi, Y. Crystal structure of anti-polysialic acid antibody single chain Fv fragment complexed with octasialic acid: Insight into the binding preference for polysialic acid. J. Biol. Chem. 2013, 288, 33784–33796. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.R.; McCoy, R.D.; Vollger, H.F.; Wilkison, N.C.; Troy, F.A. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 1971–1975. [Google Scholar] [CrossRef] [PubMed]
- Bisong, S.; Brown, R; Osim, E. Comparative effects of Rauwolfia vomitoria and chlorpromazine on social behaviour and pain. N. Am. J. Med. Sci. 2011, 3, 48–54. [Google Scholar] [CrossRef] [PubMed]










© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, C.; Nishimura, S.; Mori, A.; Niimi, Y.; Yang, Y.; Hane, M.; Kitajima, K.; Sato, C. Chlorpromazine Increases the Expression of Polysialic Acid (PolySia) in Human Neuroblastoma Cells and Mouse Prefrontal Cortex. Int. J. Mol. Sci. 2017, 18, 1123. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061123
Abe C, Nishimura S, Mori A, Niimi Y, Yang Y, Hane M, Kitajima K, Sato C. Chlorpromazine Increases the Expression of Polysialic Acid (PolySia) in Human Neuroblastoma Cells and Mouse Prefrontal Cortex. International Journal of Molecular Sciences. 2017; 18(6):1123. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061123
Chicago/Turabian StyleAbe, Chikara, Saki Nishimura, Airi Mori, Yuki Niimi, Yi Yang, Masaya Hane, Ken Kitajima, and Chihiro Sato. 2017. "Chlorpromazine Increases the Expression of Polysialic Acid (PolySia) in Human Neuroblastoma Cells and Mouse Prefrontal Cortex" International Journal of Molecular Sciences 18, no. 6: 1123. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061123