Translational Development and Application of (1→3)-β-d-Glucan for Diagnosis and Therapeutic Monitoring of Invasive Mycoses
Abstract
:1. Introduction
2. (1→3)-β-d-Glucan (BDG) in Serum
2.1. Candidiasis
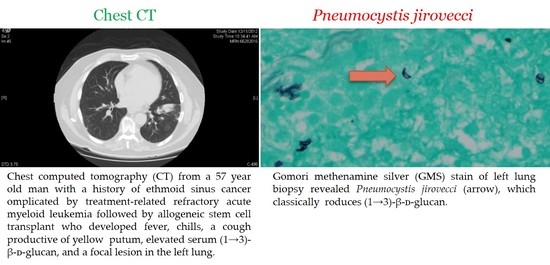
2.2. Pneumocystis jirovecii Pneumonia
2.3. Aspergillosis
3. BDG in Bronchoalveolar Lavage (BAL)
3.1. Candida Pneumonia
3.2. Invasive Pulmonary Aspergillosis
4. BDG in Cerebrospinal Fluid (CSF)
4.1. Hematogenous Candida Meningoencephalitis
4.2. Cryptococcal Meningitis
4.3. Aspergillus Ventriculitis
4.4. Exserohilum Rostratum Meningitis
4.5. Coccidioidal Meningitis
5. Conclusions and Future Directions
Conflicts of Interest
References
- Georgopapadakou, N.H.; Tkacz, J.S. The fungal cell wall as a drug target. Trends Microbiol. 1995, 3, 98–104. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrieres, V. Molecular interactions of β-(1→3)-glucans with their receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Tanaka, S.; Oda, T.; Uemura, Y.; Aketagawa, J.; Hashimoto, Y. Purification and characterization of a (1→3)-β-d-glucan-binding protein from horseshoe crab (Tachypleus tridentatus) amoebocytes. Carbohydr. Res. 1996, 295, 103–116. [Google Scholar] [PubMed]
- Pickering, J.W.; Sant, H.W.; Bowles, C.A.; Roberts, W.L.; Woods, G.L. Evaluation of a (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005, 43, 5957–5962. [Google Scholar] [CrossRef] [PubMed]
- Hector, R.F. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 1993, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, Z.; Mattiuzzi, G.; Estey, E.; Kantarjian, H.; Saeki, F.; Ridge, R.J.; Ketchum, P.A.; Finkelman, M.A.; Red, J.H.; Ostrosky-Zeichner, L.; et al. β-d-glucan as a diagnostic adjunct for invasive fungal infections: Validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 2004, 39, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cuétara, M.S.; Alhambra, A.; Moragues, M.D.; González-Elorza, E.; Pontón, J.; del Palacio, A. Detection of (1→3)-β-d-glucan as an adjunct to diagnosis in a mixed population with uncommon proven invasive fungal diseases or with an unusual clinical presentation. Clin. Vaccine Immunol. 2009, 16, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Rhein, J.; Bahr, N.C.; Morawski, B.M.; Schutz, C.; Zhang, Y.; Finkelman, M.; Meya, D.B.; Meintjes, G.; Boulware, D.G. Detection of High Cerebrospinal Fluid Levels of (1→3)-β-d-Glucan in Cryptococcal Meningitis. Open Forum. Infect. Dis. 2014, 1, ofu105. [Google Scholar] [CrossRef] [PubMed]
- Malani, A.N.; Singal, B.; Wheat, L.J.; al Sous, O.; Summons, T.A.; Durkin, M.M.; Pettit, A.C. (1→3)-β-d-glucan in cerebrospinal fluid for diagnosis of fungal meningitis associated with contaminated methylprednisolone injections. J. Clin. Microbiol. 2015, 53, 799–803. [Google Scholar] [CrossRef] [PubMed]
- De Vlieger, G.; Lagrou, K.; Maertens, J.; Verbeken, E.; Meersseman, W.; van Wijngaerden, E. β-d-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: An autopsy-based study. J. Clin. Microbiol. 2011, 49, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Prattes, J.; Spiess, B.; Wagner, J.; Prueller, F.; Raggam, R.B.; Posch, V.; Duettmann, W.; Hoenigl, K.; Wolfler, A. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 2014, 52, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Ahmad, A.; Khare, V.; Kumar, A.; Banerjee, G.; Verma, N.; Singh, M. Comparative evaluation of pan-fungal real-time PCR, galactomannan and (1→3)-β-d-glucan assay for invasive fungal infection in paediatric cancer patients. Mycoses 2017, 60, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Calandra, T. Early diagnosis of invasive mould infections and disease. J. Antimicrob. Chemother. 2017, 72, i19–i28. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. Current challenges in the diagnosis of fungal infections. Methods Mol. Biol. 2017, 1508, 3–15. [Google Scholar] [PubMed]
- Badiee, P.; Hashemizadeh, Z.; Ramzi, M.; Karimi, M.; Mohammadi, R. Non-Invasive methods to diagnose fungal infections in pediatric patients with hematologic disorders. Jundishapur. J. Microbiol. 2016, 9, e41573. [Google Scholar] [CrossRef] [PubMed]
- Boch, T.; Spiess, B.; Cornely, O.A.; Vehreschild, J.J.; Rath, P.M.; Steinmann, J.; Heinz, W.J.; Hahn, J.; Krause, S.W.; Kiehl, M.G.; et al. Diagnosis of invasive fungal infections in haematological patients by combined use of galactomannan, 1,3-β-d-glucan, Aspergillus PCR, multifungal DNA-microarray, and Aspergillus azole resistance PCRs in blood and bronchoalveolar lavage samples: Results of a prospective multicentre study. Clin. Microbiol. Infect. 2016, 22, 862–868. [Google Scholar] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Perlin, D.S.; Muldoon, E.G.; Colombo, A.L.; Chakrabarti, A.; Richardson, M.D.; Sorrell, T.C. Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerg. Infect. Dis. 2017, 23, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Pazos, C.; Moragues, M.D.; Quindós, G.; Pontón, J.; del Palacio, A. Diagnostic potential of (1,3)-β-d-glucan and anti-Candida albicans germ tube antibodies for the diagnosis and therapeutic monitoring of invasive candidiasis in neutropenic adult patients. Rev. Iberoam. Micol. 2006, 23, 209–215. [Google Scholar] [CrossRef]
- Sims, C.R.; Jaijakul, S.; Mohr, J.; Rodriguez, J.; Finkelman, M.; Ostrosky-Zeichner, L. Correlation of clinical outcomes with β-glucan levels in patients with invasive candidiasis. J. Clin. Microbiol. 2012, 50, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Jaijakul, S.; Vazquez, J.A.; Swanson, R.N.; Ostrosky-Zeichner, L. (1,3)-β-d-glucan as a prognostic marker of treatment response in invasive candidiasis. Clin. Infect. Dis. 2012, 55, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Doern, C.D. β-d-glucan testing is important for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2013, 51, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; Wang, S.H.; Liang, S.X.; Jiang, W.X.; Luo, D.D.; Huang, D.H. The screening performance of Serum 1,3-β-d-Glucan in patients with invasive fungal diseases: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0131602. [Google Scholar] [CrossRef] [PubMed]
- Grilo, V.; Pereira, A. Pneumocystis pneumonia in 107 HIV infected patients admitted to the department of infectious diseases at Santa Maria Hospital, Lisbon (2002–2013). Acta Med. Port. 2016, 29, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, R.; Phillips, B.; Wilne, S. Guidelines for the prophylaxis of Pneumocystis jirovecii Pneumonia (PJP) in children with solid tumors. J. Pediatr. Hematol. Oncol. 2017, 39, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; de Assis, Â.; Luz, E.A.; Lyra, L.; Toro, I.F.; Seabra, J.C.; Daldin, D.H.; Marcalto, T.J.; Galasso, M.T.; Macedo, R.F.; et al. Detection of Pneumocystis jirovecii by nested PCR in HIV-negative patients with pulmonary disease. Rev. Iberoam. Micol. 2017, 34, 83–88. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Backx, M.; Barnes, R.A. Diagnosis and management of Pneumocystis jirovecii infection. Expert Rev. Anti. Infect. Ther. 2017, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Meissner, A.; Goßmann, A.; Brockmann, M.; Schildgen, V.; Schildgen, O. Pneumocystis jirovecii-induced chronic interstitial lung disease in Waldenström's macroglobulinemia. Future Microbiol. 2017, 12, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Meliani, L.; Develoux, M.; Marteau-Miltgen, M.; Magne, D.; Barbu, V.; Poirot, J.L.; Roux, P. Real time quantitative PCR assay for Pneumocystis jirovecii detection. J. Eukaryot. Microbiol. 2003, 50, 651. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.F.; Lindley, A.R.; Ambrose, H.E.; Aliouat-Denis, C.M.; Wakefield, A.E. Multilocus genotyping of Pneumocystis jirovecii from adult HIV-infected patients with Pneumocystis pneumonia. J. Eukaryot. Microbiol. 2003, 50, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S.; Hasegawa, N.; Kobayashi, S.; Yamada, W.; Nishimura, T.; Takeuchi, T.; Ishizaka, A. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest 2007, 131, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; Wagner, D. β-d-Glucan kinetics for the assessment of treatment response in Pneumocystis jirovecii pneumonia. Clin. Microbiol. Infect. 2011, 17, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Jahrsdoerfer, R.A.; Ejercito, V.S.; Johns, M.M.; Cantrell, R.W.; Sydnor, J.B. Aspergillosis of the nose and paranasal sinuses. Am. J. Otolaryngol. 1979, 1, 6–14. [Google Scholar] [CrossRef]
- Milroy, C.M.; Blanshard, J.D.; Lucas, S.; Michaels, L. Aspergillosis of the nose and paranasal sinuses. J. Clin. Pathol. 1989, 42, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Jeanvoine, A.; Rocchi, S.; Reboux, G.; Grenouillet, F.; Benassarou, M.; Chirouze, C.; Millon, L. Sinus aspergillosis due to an azole-resistant Aspergillus fumigatus strain carrying the TR34/L98H mutation in immunocompetent host. Infect. Dis. 2016, 48, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Trief, D.; Gray, S.T.; Jakobiec, F.A.; Durand, M.L.; Fay, A.; Freitag, S.K.; Lee, N.G.; Lefebvre, D.R.; Holbrook, E.; Bleier, B.; et al. Invasive fungal disease of the sinus and orbit: A comparison between mucormycosis and Aspergillus. Br. J. Ophthalmol. 2016, 100, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Veys, P.; Malech, H.; Cowan, M.J. Chronic granulomatous disease: Lessons from a rare disorder. Biol. Blood Marrow Transplant. 2011, 17, S123–S131. [Google Scholar] [CrossRef] [PubMed]
- Aller-García, A.I.; Castro-Méndez, C.; Alastruey-Izquierdo, A.; Marín-Martínez, E.M.; Breval, I.Z.; Couto-Caro, C.; Lopez-Marin, J.C.; Pena-Grinan, N.; Ruiz de Pipaon, M.; Romero-Mijias, A.M.; et al. Case series study of invasive pulmonary aspergillosis. Mycopathologia 2016, 182, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Geltner, C.; Lass-Flörl, C. Invasive pulmonary Aspergillosis in organ transplants—Focus on lung transplants. Respir. Investig. 2016, 54, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Trof, R.J.; Beishuizen, A.; Debets-Ossenkopp, Y.J.; Girbes, A.R.; Groeneveld, A.B. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007, 33, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Bulpa, P.; Dive, A.; Sibille, Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 30, 782–800. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W.; Aguilar-Zapata, D.; Petraitis, V.; Walsh, T.J. Diagnosis, classification, and therapeutic interventions for sinopulmonary Aspergillosis. Expert. Rev. Respir. Med. 2017, 11, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Morrissey, C.O.; Liew, D.; Bohensky, M.A.; Chen, S.C.; Gilroy, N.M.; Milliken, S.T.; Szer, J.; Slavin, M.A. Is a biomarker-based diagnostic strategy for invasive aspergillosis cost effective in high-risk haematology patients? Med. Mycol. 2017, 55, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Trullas, J.C.; Cervera, C.; Benito, N.; de la Bellacasa, J.P.; Agustí, C.; Rovira, M.; Mas, A.; Navasa, M.; Cofan, F.; Ricart, M.J.; et al. Invasive pulmonary aspergillosis in solid organ and bone marrow transplant recipients. Transplant. Proc. 2005, 37, 4091–4093. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Bretagne, S. Challenges in microbiological diagnosis of invasive Aspergillus infections. F1000Res 2017, 7, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Letscher-Bru, V.; Oprea, C.; Lioure, B.; Waller, J.; Campos, F.; Villard, O.; Liu, K.L.; Natarajan-Ame, S.; Lutz, P.; et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 2002, 20, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Verhaegen, J.; Demuynck, H.; Brock, P.; Verhoef, G.; Vandenberghe, P.; Van Eldere, J.; Verbist, L.; Boogaerts, M. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive Aspergillosis. J. Clin. Microbiol. 1999, 37, 3223–3228. [Google Scholar] [PubMed]
- Kauffmann-Lacroix, C.; Arvier, M.; Charron, M.; Rodier, M.H.; Vassault, A. Detection of Aspergillus antigen galactomannan using ELISA method: Validation of the performances of the method for accreditation. J. Mycol. Med. 2013, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Siemann, M.; Koch-Dörfler, M. The Platelia Aspergillus ELISA in diagnosis of invasive pulmonary aspergilosis (IPA). Mycoses 2001, 44, 266–272. [Google Scholar] [PubMed]
- Pazos, C.; Pontón, J.; del Palacio, A. Contribution of (1→3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: A comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 2005, 43, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Senn, L.; Robinson, J.O.; Schmidt, S.; Knaup, M.; Asahi, N.; Satomura, S.; Matsuura, S.; Duvoisin, B.; Bille, J.; Calandra, T.; et al. 1,3-β-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 2008, 46, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Al-Ramadi, B.; Finkelman, M.; Hedstrom, U.; Kristensen, J.; Ali-Zadeh, H.; Klingspor, L. Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. J. Med. Microbiol. 2008, 57, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.D.; Fisher, B.T.; Castagnola, E.; Groll, A.H.; Steinbach, W.J.; Zaoutis, T.E.; Negeri, Z.F.; Beyene, J.; Phillips, B.; et al. Galactomannan, β-d-Glucan, and polymerase chain reaction-based assays for the diagnosis of invasive fungal disease in Pediatric cancer and hematopoietic stem cell transplantation: A systematic review and meta-analysis. Clin. Infect. Dis. 2016, 63, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Sulahian, A.; Porcher, R.; Bergeron, A.; Touratier, S.; Raffoux, E.; Menotti, J.; Derouin, F.; Ribaud, P. Use and limits of (1→3)-β-d-glucan assay (Fungitell), compared to galactomannan determination (Platelia Aspergillus), for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 2014, 52, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, W.; Risslegger, B.; Willinger, B.; Hoenigl, M.; Bucher, B.; Eschertzhuber, S.; Lass-Florl, C. Bronchoalveolar Lavage Fluid (1,3)β-d-Glucan for the Diagnosis of Invasive Fungal Infections in Solid Organ Transplantation: A Prospective Multicenter Study. Transplantation 2015, 99, e140–e144. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, S.; Don, M.; Schillinger, W.; Rüchel, R. Candida pneumonia in patients without definitive immunodeficiency. Mycoses 2002, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Delisle, M.S.; Williamson, D.R.; Perreault, M.M.; Albert, M.; Jiang, X.; Heyland, D.K. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J. Crit. Care 2008, 23, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Su, K.C.; Chou, K.T.; Hsiao, Y.H.; Tseng, C.M.; Su, V.Y.; Lee, Y.C.; Perng, D.W.; Kou, Y.R. Measuring (1,3)-β-d-glucan in tracheal aspirate, bronchoalveolar lavage fluid, and serum for detection of suspected Candida pneumonia in immunocompromised and critically ill patients: A prospective observational study. BMC Infect. Dis. 2017, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- Swoboda-Kopeć, E.; Sikora, M.; Piskorska, K.; Gołaś, M.; Netsvyetayeva, I.; Przybyłowska, D.; Mierzwinska-Nastalaska, E. Diagnosis of Invasive Pulmonary Aspergillosis. Adv. Exp. Med. Biol. 2017, 944, 27–33. [Google Scholar] [PubMed]
- Tejeda, M.I.; Salso, S.; Barberán, J. [Invasive pulmonary aspergillosis in non-neutropenic patients]. Rev. Esp. Quimioter. 2016, 29, 56–58. [Google Scholar] [PubMed]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive summary: Practice guidelines for the diagnosis and management of aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Petraitiene, R.; Petraitis, V.; Bacher, J.D.; Finkelman, M.A.; Walsh, T.J. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1→3)-β-d-glucan in experimental invasive pulmonary aspergillosis. Med. Mycol. 2015, 53, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.R.; Vallabhajosyula, S.; Velez, M.G.; Fedorko, D.P.; van Raden, M.J.; Gea-Banacloche, J.C.; Lionakis, M.S. The utility of bronchoalveolar lavage β-d-glucan testing for the diagnosis of invasive fungal infections. J. Infect. 2014, 69, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Zhang, Y.; Finkelman, M.A.; Pappagianis, D.; Clemons, K.V.; Martinez, M. Cerebrospinal Fluid (1,3)-β-d-Glucan Testing Is Useful in Diagnosis of Coccidioidal Meningitis. J. Clin. Microbiol. 2016, 54, 2707–2710. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; Thakur, K.T.; Lee, R.; Watkins, T.; Pardo, C.A.; Carson, K.A.; Markley, B.; Finkelman, M.A.; Marr, J.A.; Roos, K.L.; et al. Utility of measuring (1,3)-β-d-glucan in cerebrospinal fluid for diagnosis of fungal central nervous system infection. J. Clin. Microbiol. 2015, 53, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Morgand, M.; Rammaert, B.; Poirée, S.; Bougnoux, M.E.; Tran, H.; Kania, R.; Chretien, F.; Jouvion, G.; Lortholary, O. Chronic invasive aspergillus sinusitis and otitis with meningeal extension successfully treated with voriconazole. Antimicrob. Agents Chemother. 2015, 59, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Cornu, M.; Goudjil, S.; Kongolo, G.; Leke, A.; Poulain, D.; Chouaki, T.; Sendid, B. Evaluation of the (1,3)-β-d-glucan assay for the diagnosis of neonatal invasive yeast infections. Med. Mycol. 2017, 55, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Petraitiene, R.; Petraitis, V.; Hope, W.W.; Mickiene, D.; Kelaher, A.M.; Murray, H.A.; Mya-San, C.; Hughes, J.E.; Cotton, M.P.; Bacher, J.; et al. Cerebrospinal fluid and plasma (1→3)-β-d-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob. Agents Chemother. 2008, 52, 4121–4129. [Google Scholar] [CrossRef] [PubMed]
- Warn, P.A.; Livermore, J.; Howard, S.; Felton, T.W.; Sharp, A.; Gregson, L.; Goodwin, J.; Petraitiene, R.; Petraitis, V.; Cohen-Wolkowiez, M.; et al. Anidulafungin for neonatal hematogenous Candida meningoencephalitis: Identification of candidate regimens for humans using a translational pharmacological approach. Antimicrob. Agents Chemother. 2012, 56, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Mickiene, D.; Petraitis, V.; Petraitiene, R.; Kelaher, A.M.; Hughes, J.E.; Cotton, M.P.; Bacher, J.; Keirns, J.J.; Buell, D.; et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: Implications for echinocandin therapy in neonates. J. Infect. Dis. 2008, 197, 163–171. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Rosengart, A.; Schuetz, A.N.; Kontoyiannis, D.P.; Walsh, T.J. Mold infections of the central nervous system. N. Engl. J. Med. 2014, 371, 150–160. [Google Scholar] [CrossRef]
- Farrugia, M.K.; Fogha, E.P.; Miah, A.R.; Yednock, J.; Palmer, H.C.; Guilfoose, J. Candida meningitis in an immunocompetent patient detected through (1→3)-β-d-glucan. Int. J. Infect. Dis. 2016, 51, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, C.M.; Chen, T.K.; Toussi, S.S.; de la Mora, P.; Petraitiene, R.; Finkelman, M.A.; Walsh, T.J. (1→3)-β-d-Glucan in cerebrospinal fluid as a biomarker for Candida and Aspergillus infections of the central nervous system in pediatric patients. J. Pediatric. Infect. Dis. Soc. 2016, 5, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Barnes, R.A.; Barton, R.C.; Cleverley, J.R.; Lucas, S.B.; Kibbler, C.C.; Denning, D.W. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 2015, 15, 461–474. [Google Scholar] [CrossRef]
- Rhein, J.; Boulware, D.R.; Bahr, N.C. 1,3-β-d-glucan in cryptococcal meningitis. Lancet Infect. Dis. 2015, 15, 1136–1137. [Google Scholar] [CrossRef]
- Abassi, M.; Boulware, D.R.; Rhein, J. Cryptococcal meningitis: Diagnosis and management update. Curr. Trop. Med. Rep. 2015, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.; Wong, B.; Finkelstein, W.E.; Sternberg, S.S.; Armstrong, D. Aspergillosis of the cerebral ventricles in a heroin abuser. Case report and review of the literature. Arch. Intern. Med. 1983, 143, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Moling, O.; Lass-Floerl, C.; Verweij, P.E.; Porte, M.; Boiron, P.; Prugger, M.; Gebert, U.; Corrandini, R.; Vedovelli, C.; Rimenti, G.; et al. Case Reports. Chronic and acute Aspergillus meningitis. Mycoses 2002, 45, 504–511. [Google Scholar] [PubMed]
- Antinori, S.; Corbellino, M.; Meroni, L.; Resta, F.; Sollima, S.; Tonolini, M.; Tortorano, A.M.; Milazzo, L.; Bello, L.; Furfaro, E.; et al. Aspergillus meningitis: A rare clinical manifestation of central nervous system aspergillosis. Case report and review of 92 cases. J. Infect. 2013, 66, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Groncy, P.K.; Javahery, R.; Chai, R.Y.; Nagpala, P.; Finkelman, M.; Petraitiene, R.; Walsh, T.J. Successful treatment of Aspergillus ventriculitis through voriconazole adaptive pharmacotherapy, immunomodulation, and therapeutic monitoring of cerebrospinal fluid (1→3)-β-d-glucan. Med. Mycol. 2017, 55, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.M.; Dorratoltaj, N.; O'Dell, M.L.; Bordwine, P.; Kerkering, T.M.; Redican, K.J. Clinical Response, Outbreak Investigation, and Epidemiology of the Fungal Meningitis Epidemic in the United States: Systematic Review. Disaster. Med. Public Health Prep. 2016, 10, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Exserohilum rostratum fungal meningitis associated with methylprednisolone injections. Future Microbiol. 2013, 8, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Kainer, M.A.; Reagan, D.R.; Nguyen, D.B.; Wiese, A.D.; Wise, M.E.; Ward, J. Fungal Infections Associated with Contaminated Methylprednisolone in Tennessee. N. Engl. J. Med. 2012, 367, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Kerkering, T.M.; Grifasi, M.L.; Baffoe-Bonnie, A.W.; Bansal, E.; Garner, D.C.; Smith, J.A.; Demicco, D.D.; Schleupner, C.J.; Aldoghaither, R.A.; Savaliya, V.A.; et al. Early clinical observations in prospectively followed patients with fungal meningitis related to contaminated epidural steroid injections. Ann. Intern. Med. 2013, 158, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Pana, Z.D.; Perlin, D.S.; Kontoyiannis, D.P.; Walsh, T.J.; Roilides, E. Exserohilum infections: Review of 48 cases before the 2012 United States outbreak. Med. Mycol. 2014, 52, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; Roos, K.L.; Marr, K.A.; Neumann, H.; Trivedi, J.B.; Kimbrough, D.J.; Steiner, L.; Thakur, K.T.; harrison, D.M.; Zhang, S.X.; et al. Cerebrospinal fluid (1,3)-β-d-glucan detection as an aid for diagnosis of iatrogenic fungal meningitis. J. Clin. Microbiol. 2013, 51, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Litvintseva, A.P.; Lindsley, M.D.; Gade, L.; Smith, R.; Chiller, T.; Lyons, J.L.; Thakur, K.T.; Zhang, S.X.; Grgurich, D.E.; Kerkering, T.M.; et al. Utility of (1–3)-β-d-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections. Clin. Infect. Dis. 2014, 58, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Tager, D.; Hatch, A.; Segar, J.; Roller, B.; al Mohajer, M.; Zangeneh, T.T. Coccidioidal meningitis complicated by central nervous system vasculitis in a patient with leukemia. Med. Mycol. Case Rep. 2017, 16, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Fowler, P.; Heidari, A.; Johnson, R.H. Intrathecal Amphotericin B: A 60-Year Experience in Treating Coccidioidal Meningitis. Clin. Infect. Dis. 2016, 64, 519–524. [Google Scholar]
- Twarog, M.; Thompson, G.R. Coccidioidomycosis: Recent Updates. Semin. Respir. Crit. Care Med. 2015, 36, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.; Wang, S.; Bercovitch, R.; Bolaris, M.; van den Akker, D.; Taylor, S.; Lopez, R.; Catanzaro, A.; Cadena, J.; Chin-Hong, P.; et al. Routine CSF analysis in coccidioidomycosis is not required. PLoS ONE 2013, 8, e64249. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J.; Popa, A.S.; Catania, J.; Alexov, M.; Tsaras, G.; Lloyd, F.; Wengenack, N.L.; Enzler, M.J. Meningeal coccidioidomycosis diagnosed by real-time polymerase chain reaction analysis of cerebrospinal fluid. Mycopathologia 2011, 171, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, G.; Shelub, A.; Truong, J.; Wigen, C. Coccidioidal meningitis: Clinical presentation and management in the fluconazole era. Medicine 2010, 89, 251–284. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R.; et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: A retrospective study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef] [PubMed]
- Ananda-Rajah, M.R.; Slavin, M.A.; Thursky, K.T. The case for antifungal stewardship. Curr. Opin. Infect. Dis. 2012, 25, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Edwards, J.E.; Bryant, R.; Spellberg, B. Economic burden of mucormycosis in the United States: Can a vaccine be cost-effective? Med. Mycol. 2009, 47, 592–600. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarthy, M.W.; Petraitiene, R.; Walsh, T.J. Translational Development and Application of (1→3)-β-d-Glucan for Diagnosis and Therapeutic Monitoring of Invasive Mycoses. Int. J. Mol. Sci. 2017, 18, 1124. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061124
McCarthy MW, Petraitiene R, Walsh TJ. Translational Development and Application of (1→3)-β-d-Glucan for Diagnosis and Therapeutic Monitoring of Invasive Mycoses. International Journal of Molecular Sciences. 2017; 18(6):1124. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061124
Chicago/Turabian StyleMcCarthy, Matthew W., Ruta Petraitiene, and Thomas J. Walsh. 2017. "Translational Development and Application of (1→3)-β-d-Glucan for Diagnosis and Therapeutic Monitoring of Invasive Mycoses" International Journal of Molecular Sciences 18, no. 6: 1124. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061124