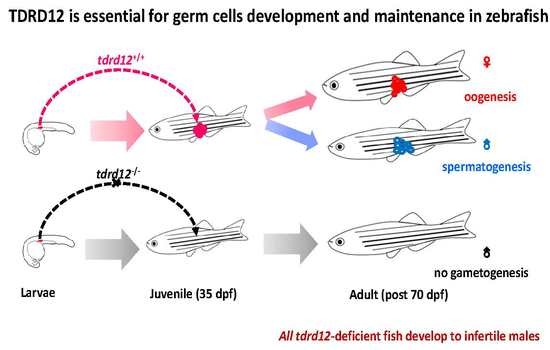
Tdrd12 Is Essential for Germ Cell Development and Maintenance in Zebrafish
Abstract
:1. Introduction
2. Results
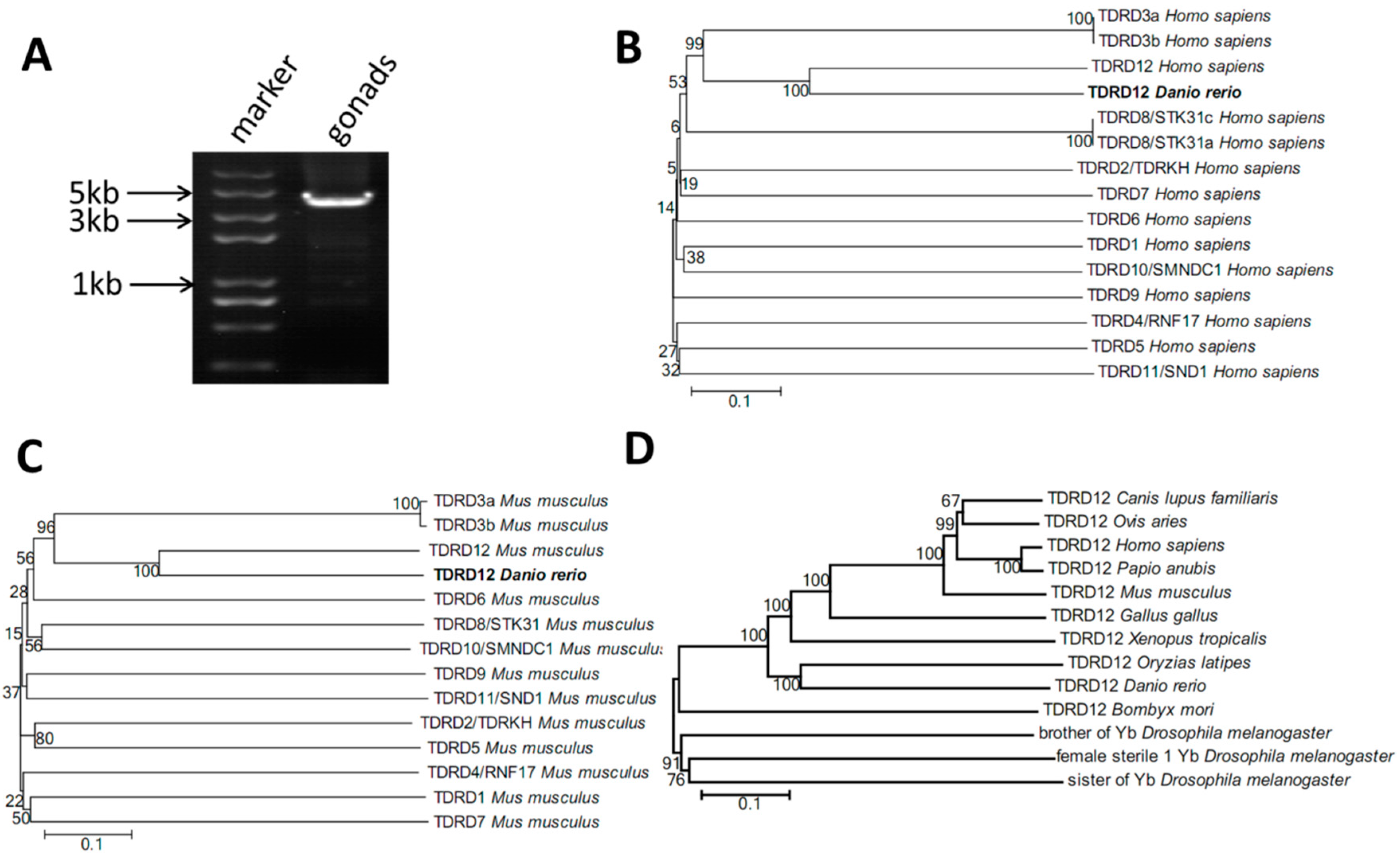
2.1. Cloning of Tdrd12 and Phylogenetic Analysis of the Tdrd12 Protein across Species
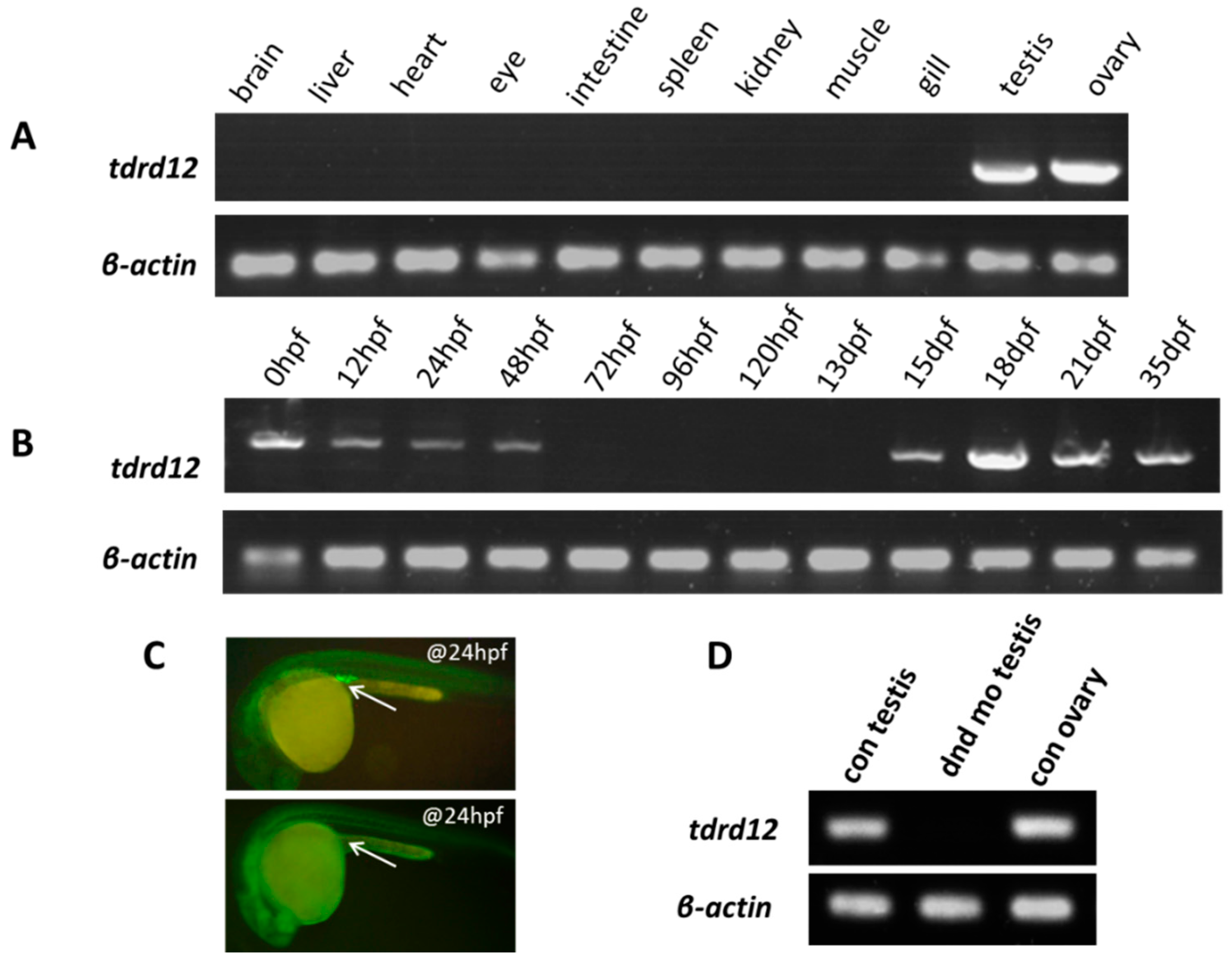
2.2. Tissue-Specific and Maternal Expression Pattern of Zebrafish tdrd12
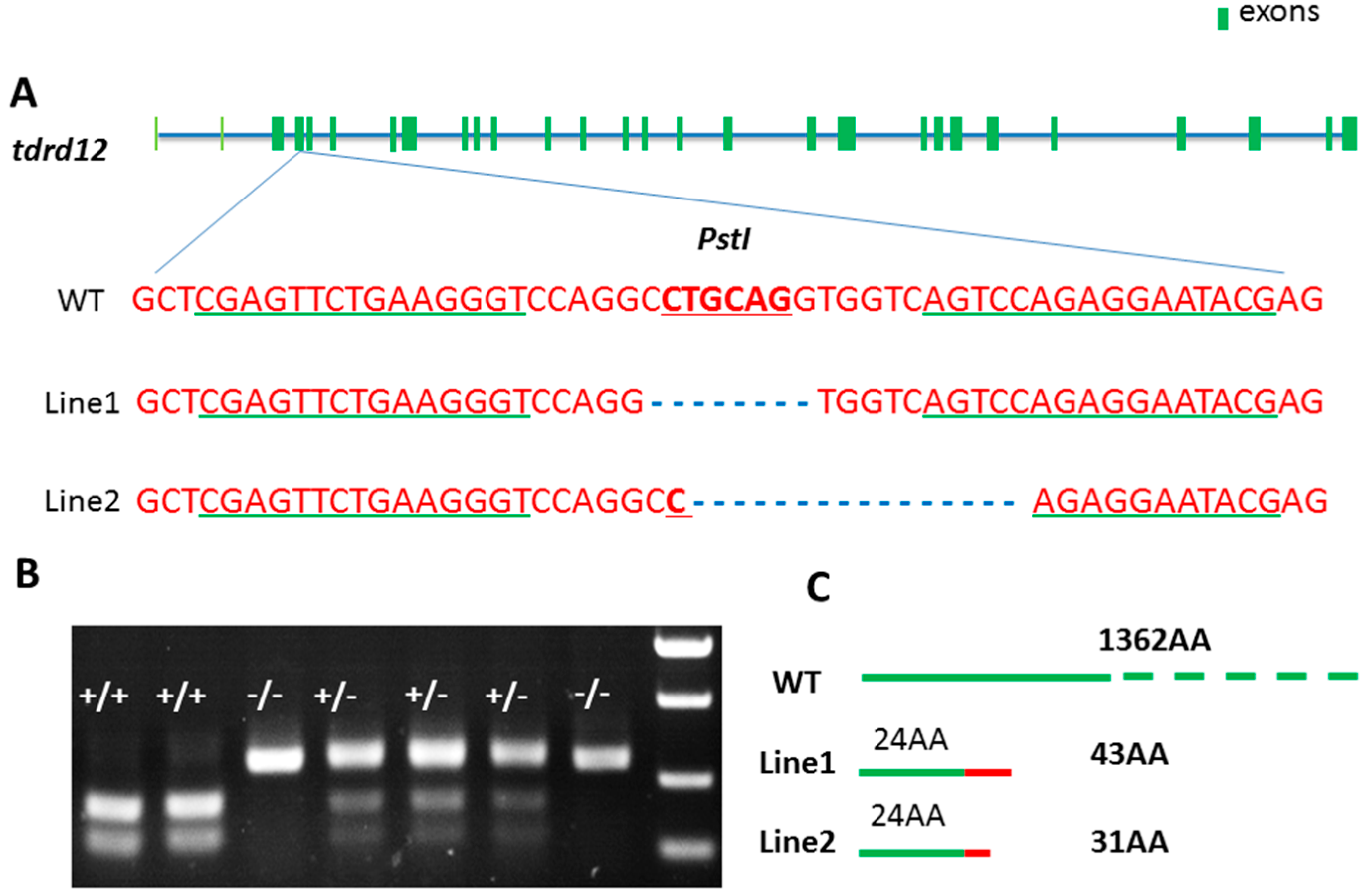
2.3. Targeted Disruption of tdrd12 Mutant Lines by TALEN
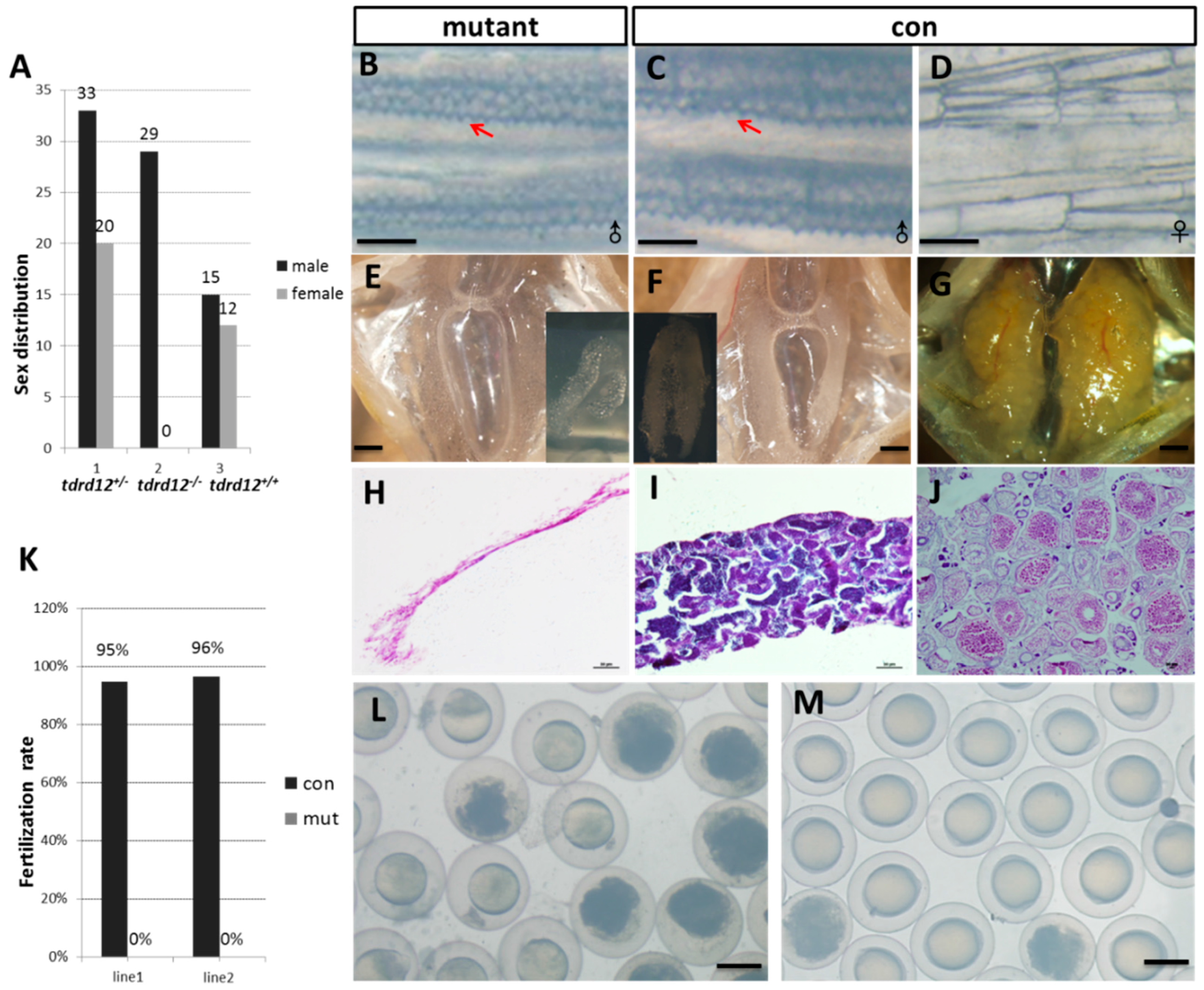
2.4. Tdrd12-Deficiency Results in Masculinization and Infertility in Zebrafish
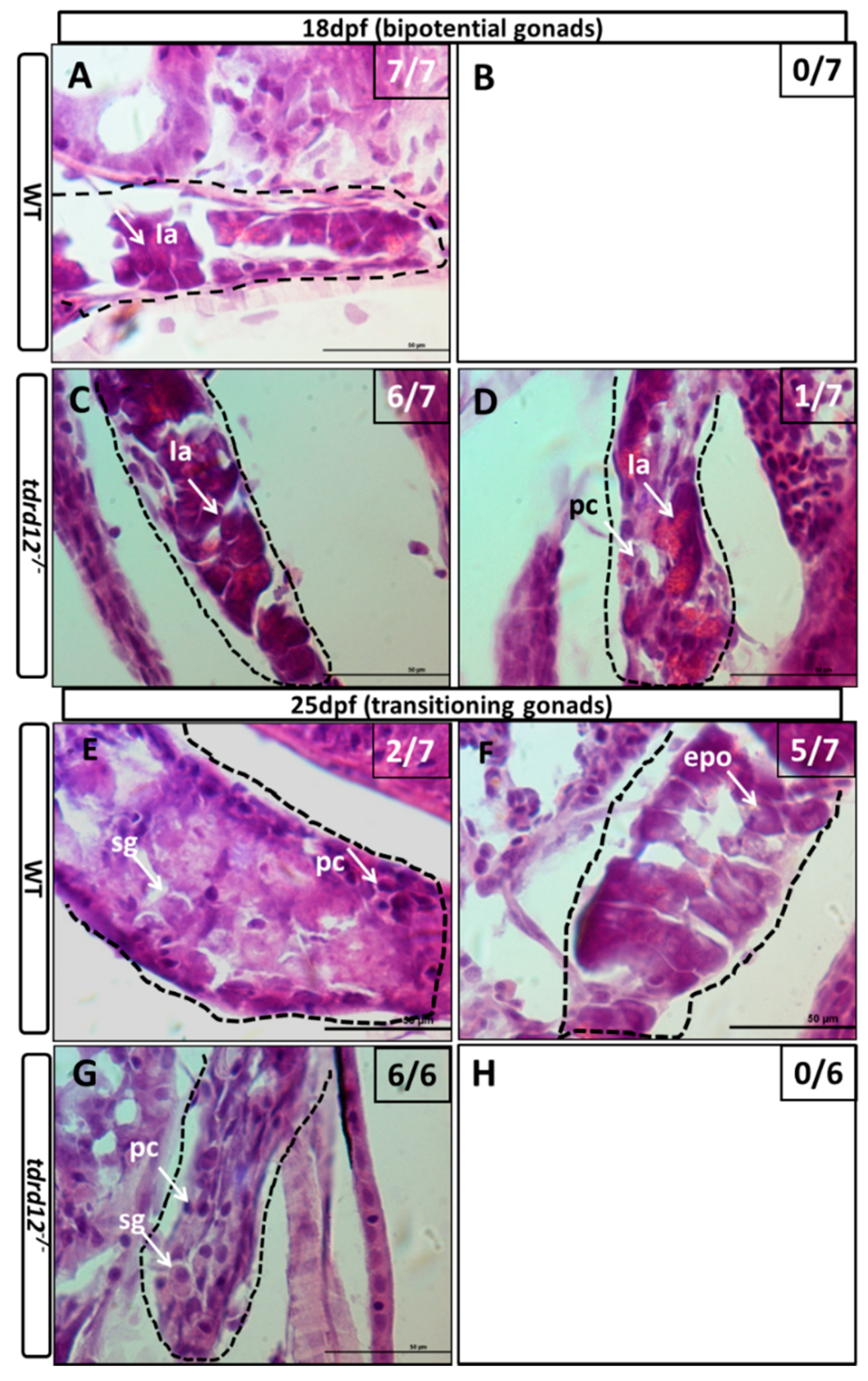
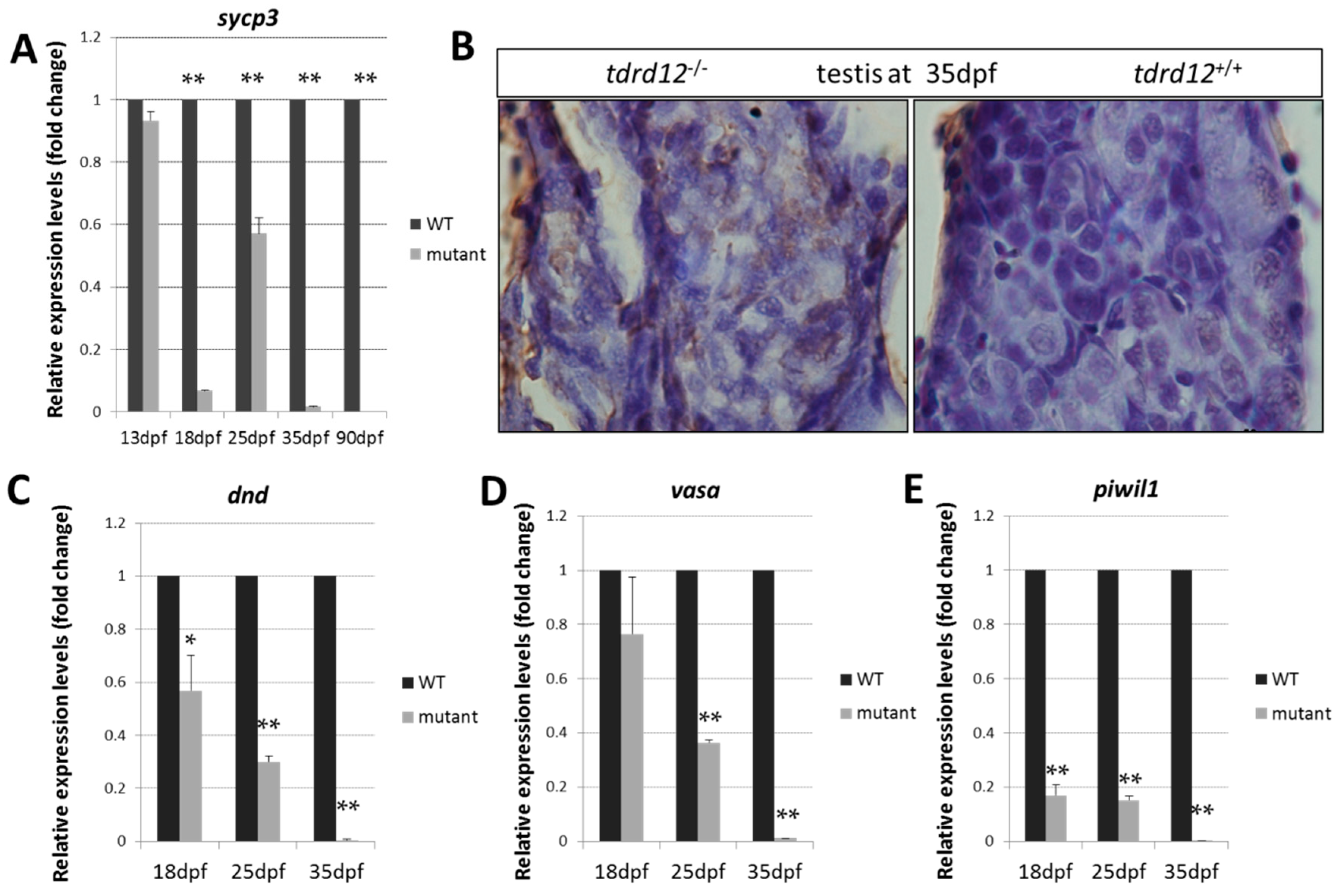
2.5. Meiosis Defects of the Germ Cells in the tdrd12 Mutants in the Gonad-Transitioning Periods
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance
4.2. tdrd12 Knock out by TALENs
4.3. RT-PCR and Quantitative Real Time PCR (Q-PCR)
4.4. Genomic DNA Isolation and Genotyping
4.5. Masculinization and Fecundability Assessments
4.6. Androgen Measurement
4.7. Anatomical and Histological Assays
4.8. In Situ Hybridization Assays
4.9. In Situ Cell Death
4.10. Fecundity Assessment
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, C.A.; High, S.K.; McCluskey, B.M.; Amores, A.; Yan, Y.L.; Titus, T.A.; Anderson, J.L.; Batzel, P.; Carvan, M.J., 3rd; Schartl, M.; et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics 2014, 198, 1291–1308. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.M.; Breyer, J.P.; Melville, D.B.; Broman, K.W.; Knapik, E.W.; Smith, J.R. An SNP-based linkage map for zebrafish reveals sex determination loci. G3 (Bethesda) 2011, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.C.; Bartfai, R.; Lim, Z.; Sreenivasan, R.; Siegfried, K.R.; Orban, L. Polygenic sex determination system in zebrafish. PLoS ONE 2012, 7, e34397. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Yamashita, M.; Kitano, T.; Iguchi, T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol. 2002, 205, 711–718. [Google Scholar] [PubMed]
- Jiang, N.; Jin, X.; He, J.; Yin, Z. The roles of follistatin 1 in regulation of zebrafish fecundity and sexual differentiation. Biol. Reprod. 2012, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mari, A.; Canestro, C.; Bremiller, R.A.; Nguyen-Johnson, A.; Asakawa, K.; Kawakami, K.; Postlethwait, J.H. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010, 6, e1001034. [Google Scholar] [CrossRef] [PubMed]
- Von Hofsten, J.; Olsson, P.-E. Zebrafish sex determination and differentiation: Involvement of FTZ-F1 genes. Reprod. Biol. Endocrinol. 2005, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Lesch, B.J.; Page, D.C. Genetics of germ cell development. Natl. Sci. Rev. 2012, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Hartung, O.; Forbes, M.M.; Marlow, F.L. Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol. Reprod. Dev. 2014, 81, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, G.; Stebler, J.; Slanchev, K.; Dumstrei, K.; Wise, C.; Lovell-Badge, R.; Thisse, C.; Thisse, B.; Raz, E. Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr. Biol. 2003, 13, 1429–1434. [Google Scholar] [CrossRef]
- Dranow, D.B.; Tucker, R.P.; Draper, B.W. Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev. Biol. 2013, 376, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tzung, K.-W.; Goto, R.; Saju, J.M.; Sreenivasan, R.; Saito, T.; Arai, K.; Yamaha, E.; Hossain, M.S.; Calvert, M.E.K.; Orbán, L. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Rep. 2015, 4, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Jin, X.; Chen, X.; He, J.; Yin, Z. Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS ONE 2015, 10, e0117824. [Google Scholar] [CrossRef] [PubMed]
- Dranow, D.B.; Hu, K.; Bird, A.M.; Lawry, S.T.; Adams, M.T.; Sanchez, A.; Amatruda, J.F.; Draper, B.W. Bmp15 is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in zebrafish. PLoS Genet. 2016, 12, e1006323. [Google Scholar] [CrossRef] [PubMed]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Houwing, S.; Berezikov, E.; Ketting, R.F. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J 2008, 27, 2702–2711. [Google Scholar] [CrossRef] [PubMed]
- Chuma, S.; Nakano, T. piRNA and spermatogenesis in mice. Philos. Trans. R. Soc. B 2012, 368, 20110338. [Google Scholar] [CrossRef] [PubMed]
- Handler, D.; Olivieri, D.; Novatchkova, M.; Gruber, F.S.; Meixner, K.; Mechtler, K.; Stark, A.; Sachidanandam, R.; Brennecke, J. A systematic analysis of Drosophila tudor domain-containing proteins identifies vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J. 2011, 30, 3977–3993. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.Y.; Lin, H. Piwi proteins and their interactors in piRNA biogenesis, germline development and gene expression. Natl. Sci. Rev. 2014, 1, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Chen, D. Tudor domain-containing proteins of Drosophila melanogaster. Dev. Growth Differ. 2012, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Chuma, S.; Hosokawa, M.; Kitamura, K.; Kasai, S.; Fujioka, M.; Hiyoshi, M.; Takamune, K.; Noce, T.; Nakatsuji, N. Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 15894–15899. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Shoji, M.; Kitamura, K.; Tanaka, T.; Noce, T.; Chuma, S.; Nakatsuji, N. Tudor-related proteins TDRD1/MTR-1, TDRD6 and TDRD7/TRAP: Domain composition, intracellular localization, and function in male germ cells in mice. Dev. Biol. 2007, 301, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Tanaka, T.; Hosokawa, M.; Reuter, M.; Stark, A.; Kato, Y.; Kondoh, G.; Okawa, K.; Chujo, T.; Suzuki, T. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev. Cell 2009, 17, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hosokawa, M.; Vagin, V.V.; Reuter, M.; Hayashi, E.; Mochizuki, A.L.; Kitamura, K.; Yamanaka, H.; Kondoh, G.; Okawa, K. Tudor domain containing 7 (tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10579–10584. [Google Scholar] [CrossRef] [PubMed]
- Saxe, J.P.; Chen, M.; Zhao, H.; Lin, H. Tdrkh is essential for spermatogenesis and participates in primary pirna biogenesis in the germline. EMBO J. 2013, 32, 1869–1885. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Houwing, S.; Kaaij, L.J.T.; Meppelink, A.; Redl, S.; Gauci, S.; Vos, H.; Draper, B.W.; Moens, C.B.; Burgering, B.M.; et al. Tdrd1 acts as a molecular scaffold for Piwi proteins and piRNA targets in zebrafish. EMBO J. 2011, 30, 3298–3308. [Google Scholar] [CrossRef] [PubMed]
- Mathioudakis, N.; Palencia, A.; Kadlec, J.; Round, A.; Tripsianes, K.; Sattler, M.; Pillai, R.S.; Cusack, S. The multiple tudor domain-containing protein TDRD1 is a molecular scaffold for mouse piwi proteins and piRNA biogenesis factors. RNA 2012, 18, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Functional Analysis of Tudor-Domain-Containing Proteins in the Zebrafish Germline. Ph.D Thesis, Hubrecht Institute, Utrecht, The Netherlands, 2012. [Google Scholar]
- Pandey, R.R.; Tokuzawa, Y.; Yang, Z.; Hayashi, E.; Ichisaka, T.; Kajita, S.; Asano, Y.; Kunieda, T.; Sachidanandam, R.; Chuma, S. Tudor domain containing 12 (TDRD12) is essential for secondary Piwi interacting RNA biogenesis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 16492–16497. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ki, B.S.; Hong, K.; Park, S.-P.; Ko, J.-J.; Choi, Y. Tudor domain containing protein TDRD12 expresses at the acrosome of spermatids in mouse testis. Asian Austral. J. Anim. 2015, 29, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Strasser, M.J.; Mackenzie, N.C.; Dumstrei, K.; Nakkrasae, L.-I.; Stebler, J.; Raz, E. Control over the morphology and segregation of zebrafish germ cell granules during embryonic development. BMC Mol. Biol. 2008, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Slanchev, K.; Stebler, J.; de la Cueva-Mendez, G.; Raz, E. Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 4074–4079. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef]
- McCurley, A.T.; Callard, G.V. Characterization of housekeeping genes in zebrafish: Male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Rupik, W.; Huszno, J.; Klag, J. Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei)—Structural and ultrastructural studies. Micron 2011, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Orban, L. Anti-müllerian hormone and 11 β-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males. Dev. Dynam. 2007, 236, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Lachke, S.A.; Alkuraya, F.S.; Kneeland, S.C.; Ohn, T.; Aboukhalil, A.; Howell, G.R.; Saadi, I.; Cavallesco, R.; Yue, Y.; Tsai, A.C. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 2011, 331, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Bowles, J.; Wilson, M.; Teasdale, R.D.; Koopman, P. Expression of the tudor-related gene Tdrd5 during development of the male germline in mice. Gene Expr. Patterns 2004, 4, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Leu, N.A.; Eckardt, S.; McLaughlin, K.J.; Wang, P.J. STK31/TDRD8, a germ cell-specific factor, is dispensable for reproduction in mice. PLoS ONE 2014, 9, e89471. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, A.; Tiedau, D.; Firooznia, A.; Müller-Reichert, T.; Jessberger, R. Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr. Biol. 2009, 19, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Ohta, H.; Abe, T.; Kurimoto, K.; Chuma, S.; Saitou, M. TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. J. Cell Biol. 2011, 192, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Saxe, J.P.; Tanaka, T.; Chuma, S.; Lin, H. Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis. Curr. Biol. 2009, 19, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Xiol, J.; Spinelli, P.; Laussmann, M.A.; Homolka, D.; Yang, Z.; Cora, E.; Couté, Y.; Conn, S.; Kadlec, J.; Sachidanandam, R.; et al. RNA clamping by vasa assembles a piRNA amplifier complex on transposon transcripts. Cell 2014, 157, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Marí, A.; Postlethwait, J.H. The role of Fanconi anemia/BRCA genes in zebrafish sex determination. In Methods in Cell Biology; Academic Press: Waltman, MA, USA, 2011; Volume 105, pp. 461–490. [Google Scholar]
- Liew, W.C.; Orban, L. Zebrafish sex: A complicated affair. Brief. Funct. Genom. 2013, 13, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, K.R.; Nusslein-Volhard, C. Germ line control of female sex determination in zebrafish. Dev. Biol. 2008, 324, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, e82. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The miqe guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Meeker, N.; Hutchinson, S.; Ho, L.; Trede, N. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. BioTechniques 2007, 43, 610–614. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Product Length |
|---|---|---|---|
| tdrd12-RT | GAGGAAACGGAGCACGTGTA | GGCTGAACTCTGCACAGGAT | 941 bp |
| tdrd12-mRNA | CGCGCGATTTTAGAAATTGGAG | TGTTCAGTCGCTGTCGCTGTCGCT | 4111 bp |
| tdrd12-genotype | GGTTTCAGTGTGATCATGGCTC | TAAACCGCTGCATTACGCACT | 296 bp |
| tdrd12-probe | TGTCGAGCTGTGGTTGAGTC | TAATACGACTCACTATAGGGACT (T7)TGTCATCGGTTTGACCAGGG | 841 bp |
| cyp11c1-real time | AACCCTGATGTGCAGGAGTG | TGAACGGTGATTCCCACAGG | 152 bp |
| amh-real time | TGACTGAACTGAGTGCGCTT | CATCTGCAGGGCTTTCAGGA | 110 bp |
| cyp19a1a-real time | TGCACAGATCCGAATTCTTCT | GCTGCGACAGGTTGTTGGTTT | 237 bp |
| ziwi (piwil1)-real time | TGACATAACAGATGGCAACCA | GCCCTCTCTCTGTTCAGGACT | 202 bp |
| dnd-real time | TGATTCCTCAACCCACCATAA | TGGACTTCATATTGCGGAGA | 202 bp |
| vasa-real time | GGGCTGCAATGTTCTGTGTG | CAGTTTGCGCATTTCTGGCT | 148 bp |
| sycp3-real time | GGCAGAAGCTGACCCAAGAT | TTTTGCACAACCCTTGCCTG | 154 bp |
| β-actin2-real time | GATGATGAAATTGCCGCACTG | ACCAACCATGACACCCTGATGT | 135 bp |
| Tudor-Containing Proteins | Chr Location/Protein | Mouse Orth. | Domains | Functions in Zebrafish | References |
|---|---|---|---|---|---|
 | 12/Tdrd1 (1176 AA) | TDRD1 | 4×Tudor, 1×zf-MYND | Germline development | [26,28] |
 | 12/Tdrd2/Tdrkh (573 AA) | TDRD2/TDRKH | 1×Tudor, 1×KHI | Not reported | |
 | 11/Tdrd3 (905 AA) | TDRD3 | 1×Tudor, 1×DUF, 1×UBA | Not reported | |
 | 9/Tdrd4/Rnf17 (1521 AA) | TDRD4/RNF17 | 5×Tudor, 1×FYVE | Not reported | |
 | 22/Tdrd5 (905 AA) | TDRD5 | 1×Tudor, 3×LOTUS | Not reported | |
 | 20/Tdrd6 (2177 AA) | TDRD6 | 7×Tudor | Germ plasm assembly | [28] |
 | 1/Tdrd7 (1079 AA) | TDRD7 | 2×Tudor, 3×LOTUS | Granule number and morphology | [31] |
 | 16/Tdrd8/Stk31 (977 AA) | TDRD8/STK31 | 1×Tudor, 1×PKc-like | Not reported | |
 | 13/Tdrd9 (1342 AA) | TDRD9 | 2×Tudor, 1×HA2, 1×HELICC | Germline development | [28] |
 | 17/Tdrd10/Smndc1 (237 AA) | TDRD10/SMNDC1 | 1×Tudor | Not reported | |
 | 4/Tdrd11/Snd1 (913 AA) | TDRD11/SND1 | 1×Tudor, 5×SNc | Not reported | |
 | 25/Tdrd12 (1362 AA) | TDRD12 | 2×Tudor, 1×DEXDc, 1×ACD | This paper |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Shu, Y.; Lou, Q.; Tian, Q.; Zhai, G.; Song, J.; Lu, S.; Yu, H.; He, J.; Yin, Z. Tdrd12 Is Essential for Germ Cell Development and Maintenance in Zebrafish. Int. J. Mol. Sci. 2017, 18, 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061127
Dai X, Shu Y, Lou Q, Tian Q, Zhai G, Song J, Lu S, Yu H, He J, Yin Z. Tdrd12 Is Essential for Germ Cell Development and Maintenance in Zebrafish. International Journal of Molecular Sciences. 2017; 18(6):1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061127
Chicago/Turabian StyleDai, Xiangyan, Yuqin Shu, Qiyong Lou, Qiang Tian, Gang Zhai, Jia Song, Suxiang Lu, Hong Yu, Jiangyan He, and Zhan Yin. 2017. "Tdrd12 Is Essential for Germ Cell Development and Maintenance in Zebrafish" International Journal of Molecular Sciences 18, no. 6: 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061127