Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Characteristics of PS NPs
2.3. Cellular Uptake of NPs and Inhibition Study
2.4. Distribution of EGF Receptors on the Cell Membrane Surface
2.5. Statistics Analysis
3. Results
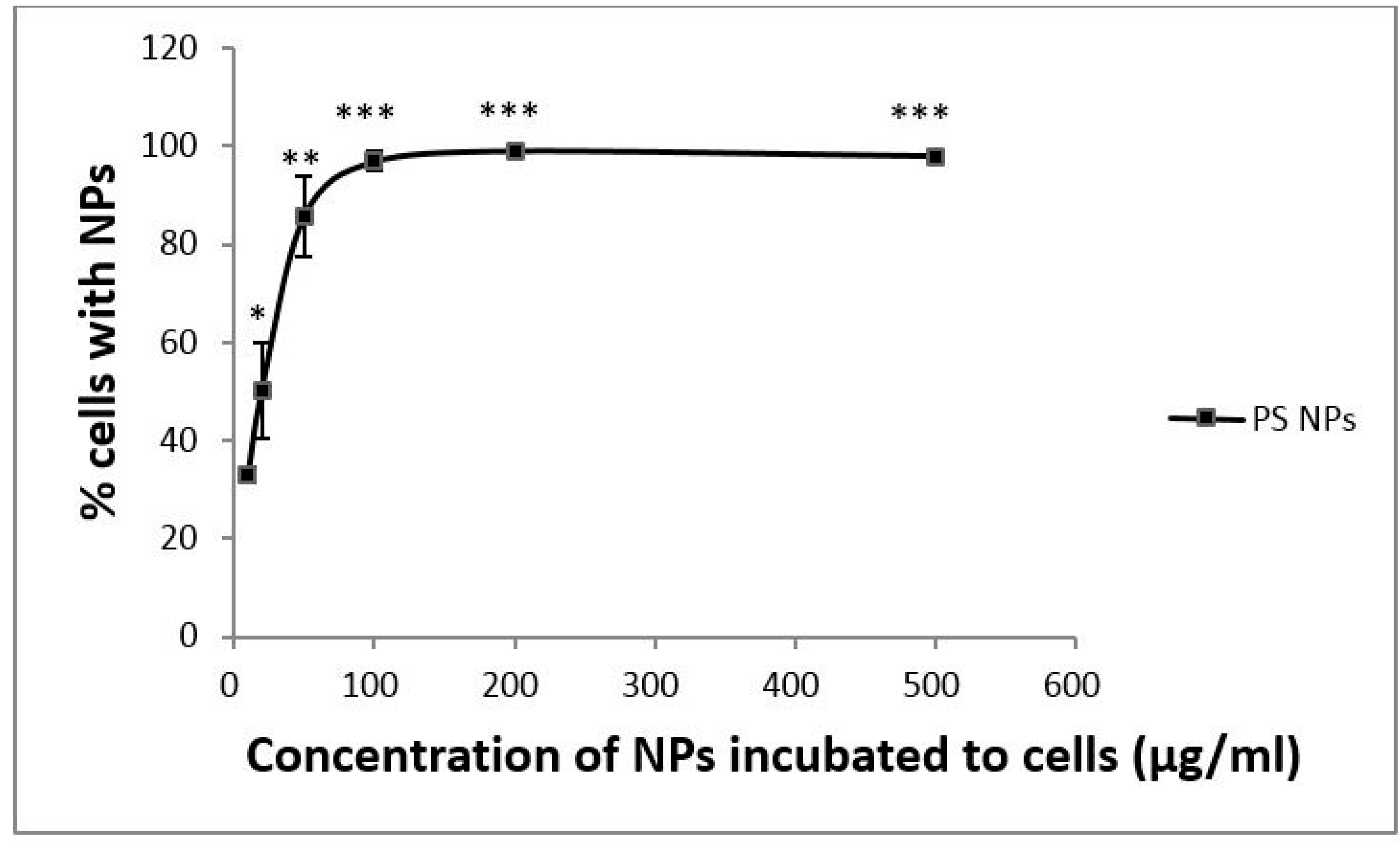
3.1. Dose-Dependent Cellular Uptake of PS NPs by A431 Cells
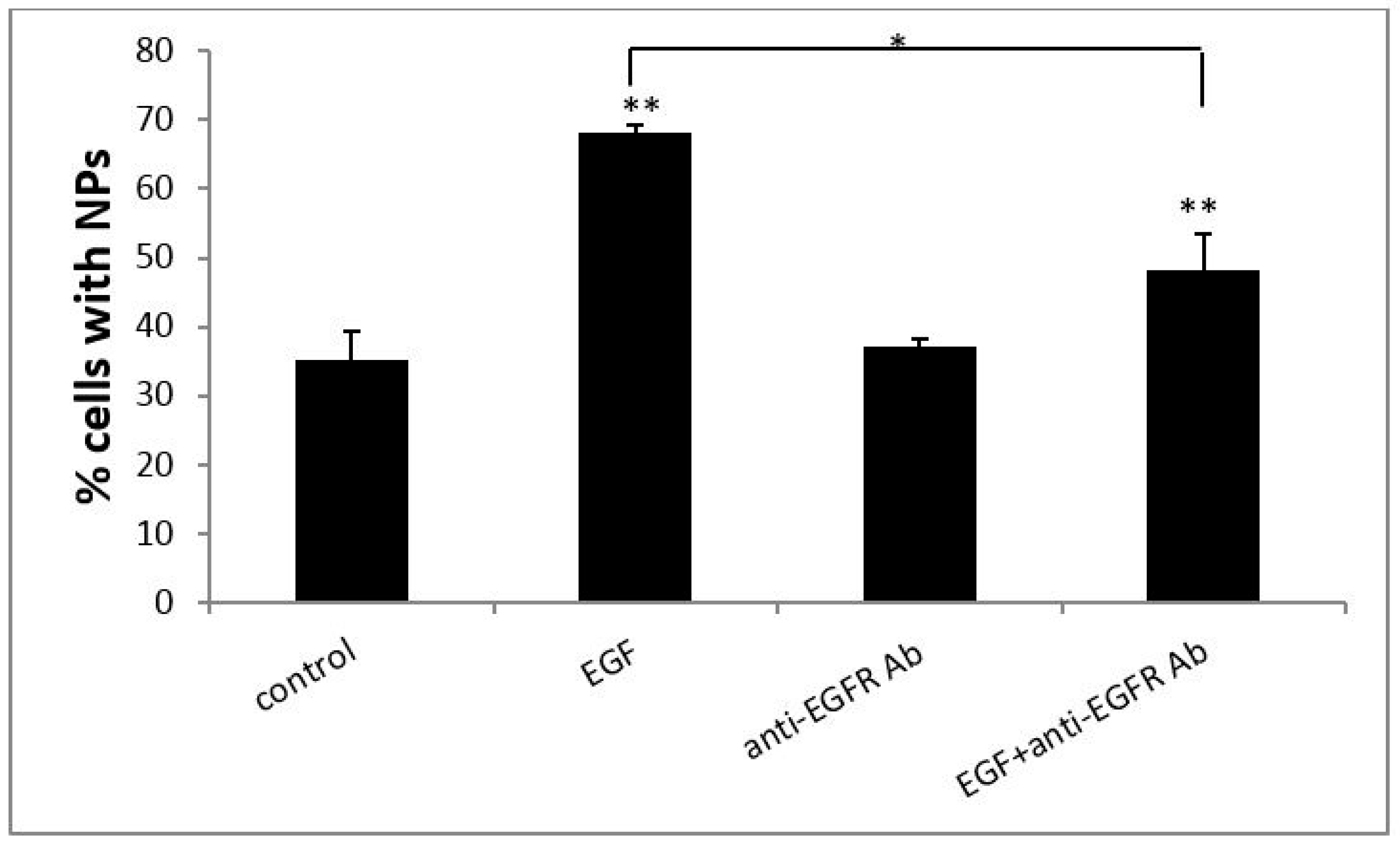
3.2. EGF Enhanced Cellular Uptake of PS NPs
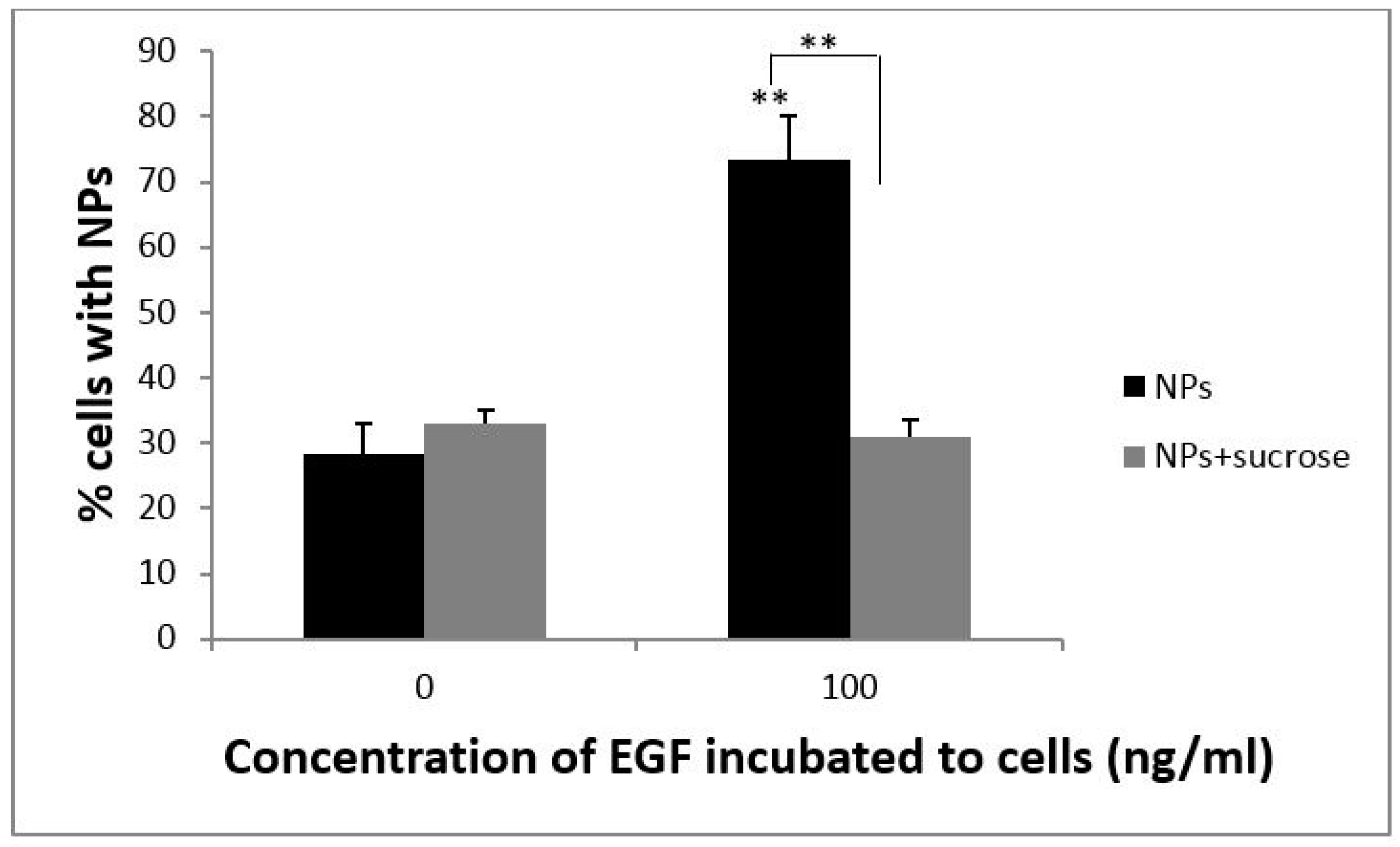
3.3. The Cellular Uptake of PS NPs by EGF Induction Occurs through Clathrin-Mediated Endocytosis
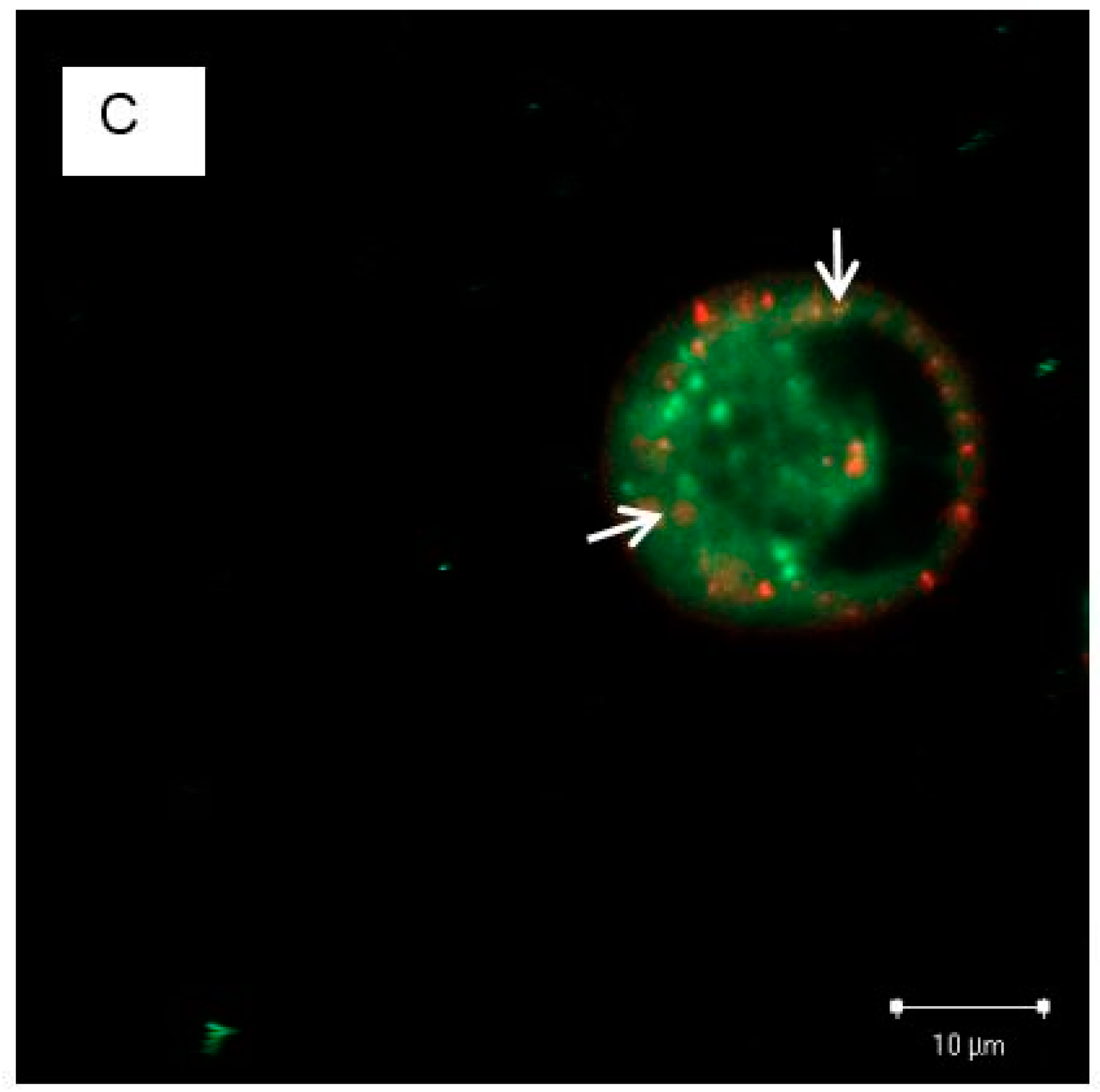
3.4. The Localization of EGFRs and PS NPs in A431 Cells with or without EGF
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cornelia, L.; Tatiana, S.; Anna, M.; Volker, M.; Katharina, L.; Ulrich, G.N.; Thomas, S. Functionalized polystyrene nanoparticles as a platform for studying bio-nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar]
- Juan, A.V.; Mariana, G.B.; Christoffer, Å.; Jeremy, C.S.; Kenneth, A.D. Quantifying size-dependent interactions between fluorescent labeled polystyrene nanoparticles and mammalian cells. J. Nanobiotechnol. 2012, 10, 39. [Google Scholar]
- Eleonore, F.; Claudia, M.; Eva, R.; Birgit, E.; Markus, A.; Thomas, R.P. Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity. Part Fibre Toxicol. 2012, 9, 26. [Google Scholar]
- Orlin, D.V.; Kaler, E.W. In situ assembly of colloidal particles into miniaturized biosensors. Langmuir 1999, 15, 3693–3698. [Google Scholar]
- Rogach, A.; Susha, A.; Caruso, F.; Sukhorukov, G.; Kornowski, A.; Kershaw, S.; Möhwald, H.; Eychmüller, A.; Weller, H. Nano- and microengineering: 3-D colloidal photonic crystals prepared from sub-µm-sized polystyrene latex spheres precoated with luminescent polyelectrolyte/Nanocrystal shells. Adv. Mater. 2000, 12, 333–337. [Google Scholar] [CrossRef]
- Andrew, K.B.; Faysal, l.; Jason, E.D.; Thomas, T.; Thomas, P.R.; Vincent, M.R. Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 2000, 404, 746–748. [Google Scholar]
- Phosphores Microsphere, Nanoparticles & Drug Delivery. Available online: http://www.phosphorex.com/1/post/2013/07/commercial-applications-use-polystyrene-nanoparticles.html (accessed on 17 April 2017).
- Johnston, H.J.; Semmler-Behnke, M.; Brown, D.M.; Kreyling, W.; Tran, L.; Stone, V. Evaluating the uptake and intracellular fate of polystyrene nanoparticles by primary and hepatocyte cell lines in vitro. Toxicol. Appl. Pharmacol. 2010, 242, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008, 2, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Geys, J.; Coenegrachts, L.; Vercammen, J.; Engelborghs, Y.; Nemmar, A.; Nemery, B.; Hoet, P.H. In vitro study of the pulmonary translocation of nanoparticles: A preliminary study. Toxicol. Lett. 2006, 160, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothe-Rutishauser, B. Different endocytotic mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Rebuma, F.; Tobias, A.O.; Heidrun, M. Identification of multiple cellular uptake pathways of polystyrene nanoparticles and factors affecting the uptake: Relevance for drug delivery system. Eur. J. Cell Biol. 2014, 93, 323–337. [Google Scholar]
- Corner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar]
- Yan, Y.; Georgina, K.S.; Jangus, P.R.J.; James, P.B.; Frank, C. Engineering particles for therapeutic delivery: Prospects and challenges. ACS Nano 2012, 6, 3663–3669. [Google Scholar] [PubMed]
- Wolfgang, Z.; Neil, A.F.; Adrian, M.R. In vitro uptake of polystyrene microspheres: Effect of particle size, cell line and cell density. J. Control. Release 2001, 71, 39–51. [Google Scholar]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [PubMed]
- Dos Santos, C.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Quantitative assessment of the comparative nanoparticles-uptake efficiency of a range of cell lines. Small 2011, 7, 3341–3349. [Google Scholar] [PubMed]
- Heather, H.; Nicole, D.; Arwyn, T.J.; Hanno, H.; Hamidreza, G.; Claus-Michael, L. Nanoparticle geometry and surface orientation influence mode of cellular uptake. ACS Nano 2013, 7, 1961–1973. [Google Scholar]
- Saha, K.; Kim, S.T.; Yan, B.; Miranda, O.R.; Alfonso, F.S.; Shlosman, D.; Rotello, V.M. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small 2013, 9, 300–305. [Google Scholar] [PubMed]
- Jong, A.K.; Christoffer, Å.; Anna, S.; Kenneth., A.D. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 2012, 7, 62–68. [Google Scholar]
- Kawamoto, T.; Sato, J.D.; Le, A.; Polikoff, J.; Sato, G.H.; Mendelsohn, J. Growth stimulation of A431 cells by epidermal growth factor: Identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc. Natl. Acad. Sci. USA 1983, 80, 1337–1341. [Google Scholar] [PubMed]
- Lim, Y.J.; Jeon, S.R.; Koh, J.M.; Wu, H.G. Tumor growth suppression and enhanced radioresponse by an exogenous epidermal growth factor in mouse xenograft models with A431 cells. Cancer Res. Treat. 2015, 47, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Masui, H.; Castro, L.; Mendelsohn, J. Consumption of EGF by A431 cells: Evidence for receptor recycling. J. Cell Biol. 1993, 120, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Chinkers, M.; McKanna, J.A.; Cohen, S. Rapid induction of morphology changes in human carcinoma cells A431 by epidermal growth factors. J. Cell. Biol. 1979, 83, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, S.H.; Spencer, B.G. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: Implications for cancer therapy. Cell Signal. 2006, 18, 2089–2097. [Google Scholar]
- Kelf, T.A.; Sreenivasan, V.K.; Sun, J.; Kim, E.J.; Goldys, E.M.; Zvyagin, A.V. Non-specific cellular uptake of surface-functionalized quantum dots. Nanotechnology 2010, 21, 285105. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Daukas, G.; Zigmond, S.H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell. Biol. 1985, 101, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Fred, S.W.; Philippe, I.H.B. Fluorescence lifetime imaging of receptor tyrosine kinase activity in cells. Curr. Biol. 1999, 9, 1127–1130. [Google Scholar]
- Bag, N.; Huang, S.; Wohland, T. Plasma membrane organization of epidermal growth factor receptor in resting and ligand-bound states. Biophys. J. 2015, 109, 1925–1936. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuc, L.T.M.; Taniguchi, A. Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis. Int. J. Mol. Sci. 2017, 18, 1301. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061301
Phuc LTM, Taniguchi A. Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis. International Journal of Molecular Sciences. 2017; 18(6):1301. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061301
Chicago/Turabian StylePhuc, Le Thi Minh, and Akiyoshi Taniguchi. 2017. "Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis" International Journal of Molecular Sciences 18, no. 6: 1301. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061301






