Two Coiled-Coil Proteins, WEB1 and PMI2, Suppress the Signaling Pathway of Chloroplast Accumulation Response that Is Mediated by Two Phototropin-Interacting Proteins, RPT2 and NCH1, in Seed Plants
Abstract
:1. Introduction
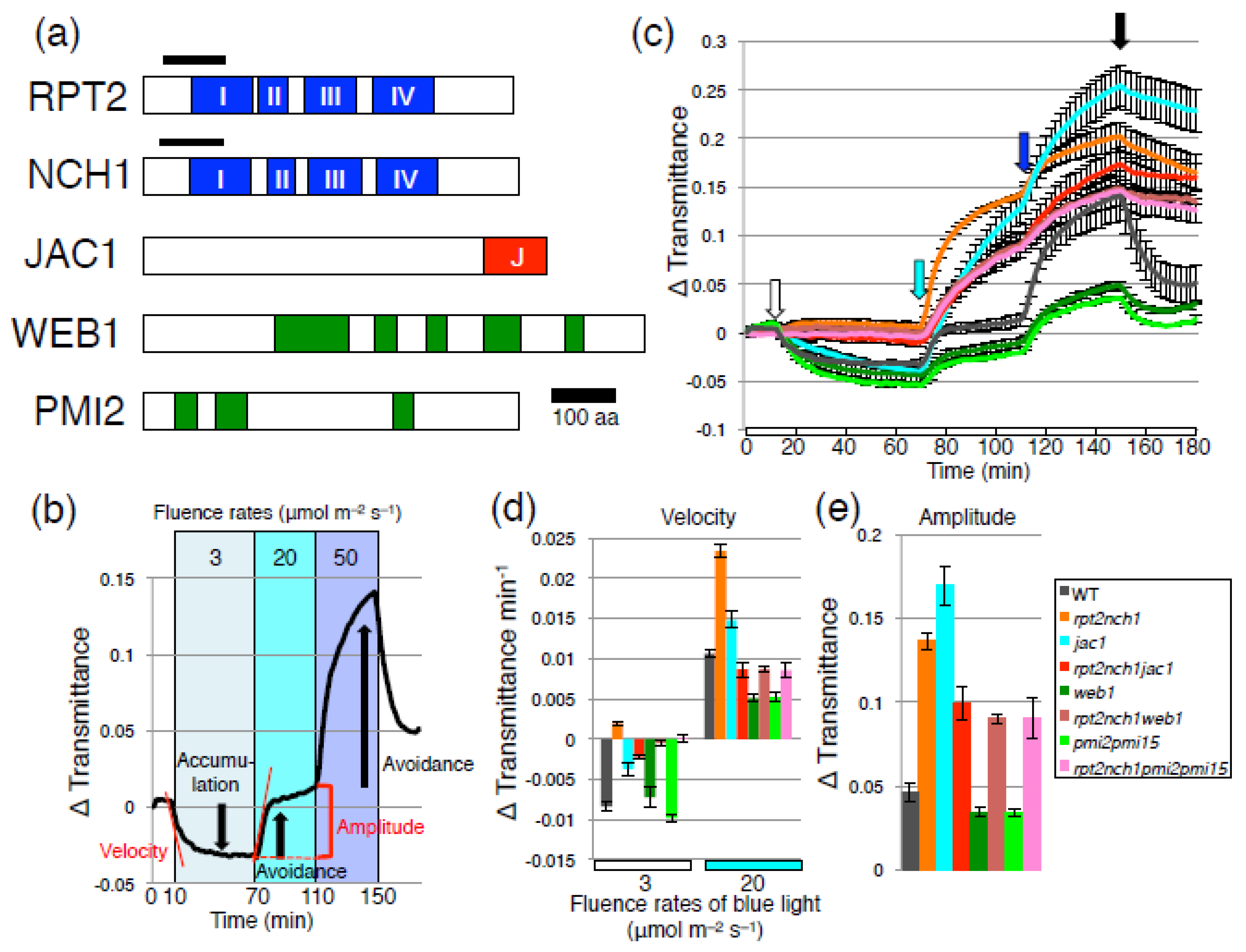
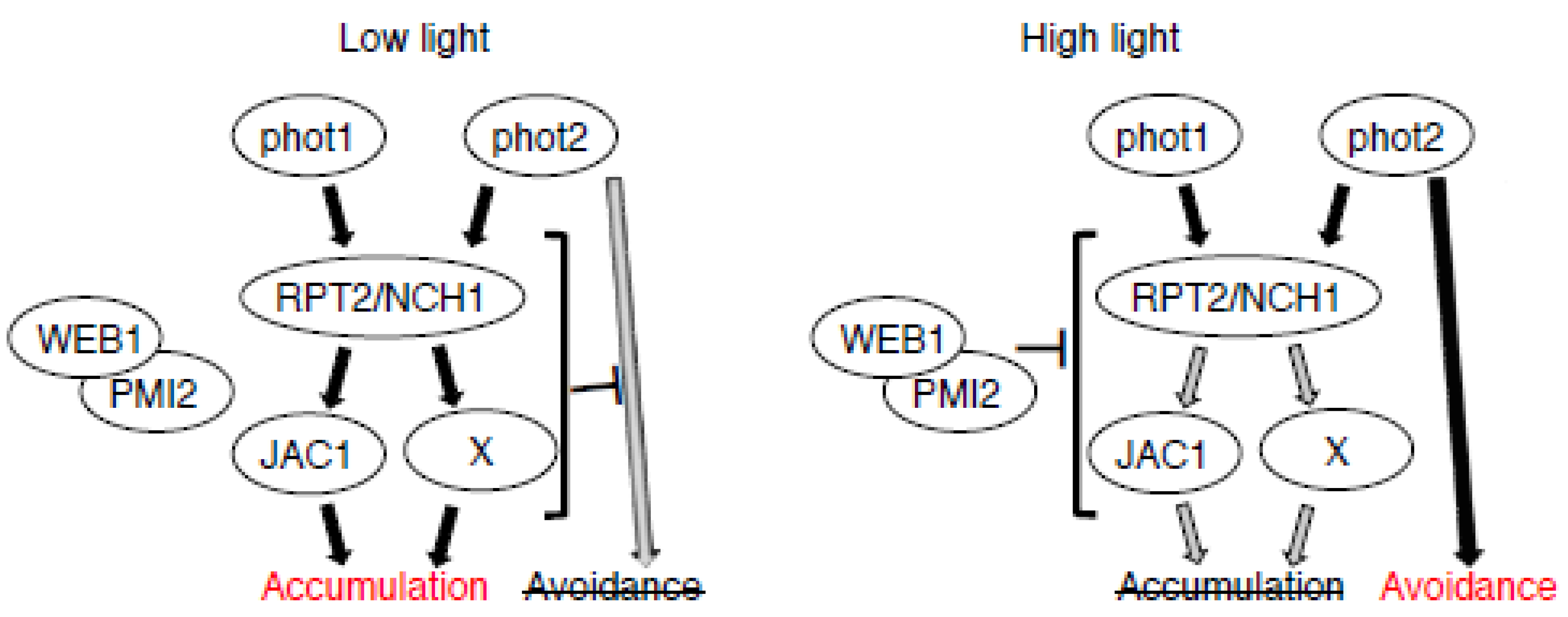
2. Results and Discussion
3. Materials and Methods
3.1. Arabidopsis Lines and the Growth Condition
3.2. Analyses of Chloroplast Photorelocation Movements
3.3. Statistical Analysis
Acknowledgements
Author Contributions
Conflicts of interest
References
- Christie, J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, N.; Wada, M. Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: Phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant Cell Physiol. 2013, 54, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Kagawa, T.; Sato, Y.; Kiyosue, T.; Wada, M. Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol. 2004, 135, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Kasahara, M.; Abe, T.; Yoshida, S.; Wada, M. Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol. 2004, 45, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, A.; Terai, M.; Ishizaki, K.; Suetsugu, N.; Tsuboi, H.; Nishihama, R.; Yamato, K.T.; Wada, M.; Kohchi, T. Phototropin encoded by a single-copy gene mediates chloroplast photorelocation movements in the liverwort Marchantia polymorpha. Plant Physiol. 2014, 166, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Li, F.W.; Rothfels, C.J.; Melkonian, M.; Villarreal, J.C.; Stevenson, D.W.; Graham, S.W.; Wong, G.K.S.; Mathews, S.; Pryer, K.M. The origin and evolution of phototropins. Front. Plant Sci. 2015, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Takemiya, A.; Sugiyama, N.; Fujimoto, H.; Tsutsumi, T.; Yamauchi, S.; Hiyama, A.; Tada, Y.; Christie, J.M.; Shimazaki, K. Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat. Commun. 2013, 4, 2094. [Google Scholar] [CrossRef] [PubMed]
- Motchoulski, A.; Liscum, E. Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 1999, 286, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Wada, T.; Ishiguro, S.; Okada, K. RPT2: A signal transducer of the phototropic response in Arabidopsis. Plant Cell 2000, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Kinoshita, T.; Takemiya, A.; Doi, M.; Shimazaki, K. Leaf positioning of Arabidopsis in response to blue light. Mol. Plant 2008, 1, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Takemiya, A.; Inoue, S.; Sakai, T.; Shimazaki, K. Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol. 2013, 54, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, N.; Takemiya, A.; Kong, S.G.; Higa, T.; Komatsu, A.; Shimazaki, K.; Kohchi, T.; Wada, M. RPT2/NCH1 subfamily of NPH3-like proteins is essential for the chloroplast accumulation response in land plants. Proc. Natl. Acad. Sci. USA 2016, 113, 10424–10429. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, N.; Kagawa, T.; Wada, M. An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 2005, 139, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kong, S.G. Analysis of chloroplast movement and relocation in Arabidopsis. Methods Mol. Biol. 2011, 774, 87–102. [Google Scholar] [CrossRef]
- Kodama, Y.; Suetsugu, N.; Kong, S.G.; Wada, M. Two interacting coiled-coil proteins, WEB1 and PMI2, maintain the chloroplast photorelocation movement velocity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 19591–19596. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, N.; Wada, M. Evolution of the cp-actin-based motility system of chloroplasts in green plants. Front. Plant Sci. 2016, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Luesse, D.R.; DeBlasio, S.L.; Hangarter, R.P. Plastid movement impaired 2, a new gene involved in normal blue-light-induced chloroplast movements in Arabidopsis. Plant Physiol. 2006, 141, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suetsugu, N.; Wada, M. Two Coiled-Coil Proteins, WEB1 and PMI2, Suppress the Signaling Pathway of Chloroplast Accumulation Response that Is Mediated by Two Phototropin-Interacting Proteins, RPT2 and NCH1, in Seed Plants. Int. J. Mol. Sci. 2017, 18, 1469. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071469
Suetsugu N, Wada M. Two Coiled-Coil Proteins, WEB1 and PMI2, Suppress the Signaling Pathway of Chloroplast Accumulation Response that Is Mediated by Two Phototropin-Interacting Proteins, RPT2 and NCH1, in Seed Plants. International Journal of Molecular Sciences. 2017; 18(7):1469. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071469
Chicago/Turabian StyleSuetsugu, Noriyuki, and Masamitsu Wada. 2017. "Two Coiled-Coil Proteins, WEB1 and PMI2, Suppress the Signaling Pathway of Chloroplast Accumulation Response that Is Mediated by Two Phototropin-Interacting Proteins, RPT2 and NCH1, in Seed Plants" International Journal of Molecular Sciences 18, no. 7: 1469. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071469



