Non-Coding RNAs as Predictive Biomarkers to Current Treatment in Metastatic Colorectal Cancer
Abstract
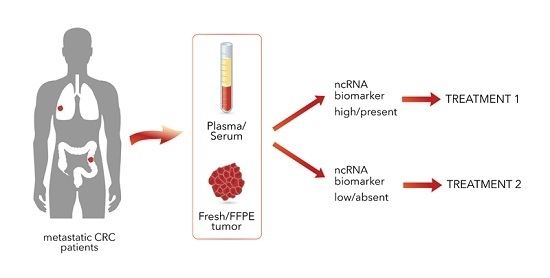
:1. Introduction
2. Non-Coding RNAs as Predictive Biomarkers to Chemotherapy in Colorectal Cancer
3. Non-Coding RNA as Predictive Biomarkers for Anti-VEGF Monoclonal Antibodies in Colorectal Cancer
4. Non-Coding RNA as Predictive Biomarkers Anti-EGFR Monoclonal Antibodies in Colorectal Cancer
5. Non-Coding RNA as Predictive Biomarkers Immunotherapy in Colorectal Cancer
6. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Tejpar, S.; Stintzing, S.; Ciardiello, F.; Tabernero, J.; Van Cutsem, E.; Beier, F.; Esser, R.; Lenz, H.J.; Heinemann, V. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the crystal and fire-3 trials. JAMA Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Moretto, R.; Masi, G.; Falcone, A. First-line therapy for mCRC—The influence of primary tumour location on the therapeutic algorithm. Nat. Rev. Clin. Oncol. 2017, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Ricard, I.; Heinemann, V.; Hegewisch-Becker, S.; Schmiegel, W.; Porschen, R.; Stintzing, S.; Graeven, U.; Arnold, D.; von Weikersthal, L.F.; et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the aio colorectal cancer study group. Ann. Oncol. 2016, 27, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Oh, S.C. Advances of targeted therapy in treatment of unresectable metastatic colorectal cancer. BioMed Res. Int. 2016, 2016, 7590245. [Google Scholar] [CrossRef] [PubMed]
- Salendo, J.; Spitzner, M.; Kramer, F.; Zhang, X.; Jo, P.; Wolff, H.A.; Kitz, J.; Kaulfuss, S.; Beissbarth, T.; Dobbelstein, M.; et al. Identification of a microRNA expression signature for chemoradiosensitivity of colorectal cancer cells, involving miRNAs-320a, -224, -132 and let7g. Radiother. Oncol. 2013, 108, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lei, W.; Fu, J.C.; Zhang, L.; Li, J.H.; Xiong, J.P. Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem. Biophys. Res. Commun. 2014, 443, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Valeri, N.; Gasparini, P.; Braconi, C.; Paone, A.; Lovat, F.; Fabbri, M.; Sumani, K.M.; Alder, H.; Amadori, D.; Patel, T.; et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA muts homolog 2 (HMSH2). Proc. Natl. Acad. Sci. USA 2010, 107, 21098–21103. [Google Scholar] [CrossRef] [PubMed]
- Karaayvaz, M.; Zhai, H.; Ju, J. miR-129 promotes apoptosis and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cell Death Dis. 2013, 4, e659. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.J.; Cai, X.J.; Li, S.J. The clinical significance of miR-429 as a predictive biomarker in colorectal cancer patients receiving 5-fluorouracil treatment. Med. Sci. Monit. 2016, 22, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ju, H.; Zhang, L.; Lu, H.; Jie, K. MicroRNA-577 suppresses tumor growth and enhances chemosensitivity in colorectal cancer. J. Biochem. Mol. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liang, X.; Cui, D.; Wu, Y.; Shi, W.; Liu, J. miR-1915 inhibits Bcl-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Mol. Carcinog. 2013, 52, 70–78. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xie, G.; Tong, J.; Peng, Y.; Huang, H.; Li, J.; Wang, N.; Liang, H. Overexpression of microrna-122 re-sensitizes 5-fu-resistant colon cancer cells to 5-fu through the inhibition of PKM2 in vitro and in vivo. Cell Biochem. Biophy. 2014, 70, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Leung, W.W.; Ng, S.S. A novel miR-203-DNMT3B-ABCG2 regulatory pathway predisposing colorectal cancer development. Mol. Carcinog. 2017, 56, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ramos, C.M.; Habr-Gama, A.; Quevedo Bde, S.; Felicio, N.M.; Bettoni, F.; Koyama, F.C.; Asprino, P.F.; Galante, P.A.; Gama-Rodrigues, J.; Camargo, A.A.; et al. Overexpression of miR-21-5p as a predictive marker for complete tumor regression to neoadjuvant chemoradiotherapy in rectal cancer patients. BMC Med. Genom. 2014, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Carames, C.; Cristobal, I.; Moreno, V.; del Puerto, L.; Moreno, I.; Rodriguez, M.; Marin, J.P.; Correa, A.V.; Hernandez, R.; Zenzola, V.; et al. MicroRNA-21 predicts response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int. J. Colorectal Dis. 2015, 30, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pickard, K.; Jenei, V.; Bullock, M.D.; Bruce, A.; Mitter, R.; Kelly, G.; Paraskeva, C.; Strefford, J.; Primrose, J.; et al. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res. 2013, 73, 6435–6447. [Google Scholar] [CrossRef] [PubMed]
- Ferracin, M.; Lupini, L.; Mangolini, A.; Negrini, M. Circulating non-coding RNA as biomarkers in colorectal cancer. Adv. Exp. Med. Biol. 2016, 937, 171–181. [Google Scholar] [PubMed]
- Chen, Q.; Xia, H.W.; Ge, X.J.; Zhang, Y.C.; Tang, Q.L.; Bi, F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Kjersem, J.B.; Ikdahl, T.; Lingjaerde, O.C.; Guren, T.; Tveit, K.M.; Kure, E.H. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol. Oncol. 2014, 8, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, K.; Bi, M.; Jiao, X.; Zhang, D.; Dong, Q. Circulating microRNA expressions in colorectal cancer as predictors of response to chemotherapy. Anticancer Drugs 2014, 25, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, Y.; Li, Y.; Ding, D. TUG1 mediates methotrexate resistance in colorectal cancer via miR-186/CPEB2 axis. Biochem. Biophys. Res. Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Wang, H.; Wang, L.; Liu, T.; Du, L.; Yang, Y.; Wang, C. MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol. Cancer Ther. 2017, 16, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Jin, L.; Zhang, J.; Yin, Y.; Quan, C.; Hu, Y.; Feng, Y.; Liu, H.; Fei, B.; Mao, Y.; et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci. Rep. 2016, 6, 23892. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.Y.; Kim, M.J.; Bae, J.M.; Koh, J.M.; Cho, N.Y.; Juhnn, Y.S.; Kim, D.; Kang, G.H. Clinical outcomes of patients with microsatellite-unstable colorectal carcinomas depend on l1 methylation level. Ann. Surg. Oncol. 2012, 19, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.T.; Chen, C.W.; Fan, Y.C.; Chang, W.C.; Lu, C.Y.; Wu, I.C.; Hsu, W.H.; Huang, C.W.; Wang, J.Y. Line-1 methylation status correlates significantly to post-therapeutic recurrence in stage III colon cancer patients receiving FOLFOX-4 adjuvant chemotherapy. PLoS ONE 2014, 10, e0123973. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kotake, M.; Bando, H.; Yamada, T.; Takemura, H.; Minamoto, T. Prognostic and predictive significance of long interspersed nucleotide element-1 methylation in advanced-stage colorectal cancer. BMC Cancer 2016, 16, 945. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.F.; Carlsen, A.L.; Heegaard, N.H.; Sorensen, F.B.; Jakobsen, A. Changes in circulating microRNA-126 during treatment with chemotherapy and bevacizumab predicts treatment response in patients with metastatic colorectal cancer. Br. J. Cancer 2015, 112, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J. Mol. Cell. Cardiol. 2016, 97, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Lv, Z.; Cao, L.; Ding, C.; Gyabaah, O.A.; Xie, H.; Zhou, L.; Wu, J.; Zheng, S. miR-126-3p suppresses tumor metastasis and angiogenesis of hepatocellular carcinoma by targeting LRP6 and PIK3R2. J. Transl. Med. 2014, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Boisen, M.K.; Dehlendorff, C.; Linnemann, D.; Nielsen, B.S.; Larsen, J.S.; Osterlind, K.; Nielsen, S.E.; Tarpgaard, L.S.; Qvortrup, C.; Pfeiffer, P.; et al. Tissue microRNAs as predictors of outcome in patients with metastatic colorectal cancer treated with first line capecitabine and oxaliplatin with or without bevacizumab. PLoS ONE 2014, 9, e109430. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alfonso, P.; Feliu, J.; Garcia-Carbonero, R.; Gravalos, C.; Guillen-Ponce, C.; Sastre, J.; Garcia-Foncillas, J. Is regorafenib providing clinically meaningful benefits to pretreated patients with metastatic colorectal cancer? Clin. Transl. Oncol. 2016, 18, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, B.; Cao, L.; Zhu, F.; Chen, B.; Lv, H.; Fan, X.; Han, L.; Bie, L.; Cao, X.; et al. Direct binding of microRNA-21 pre-element with regorafenib: An alternative mechanism for anti-colorectal cancer chemotherapy? J. Mol. Graph. Model. 2017, 73, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, J.; Faltejskova, P.; Nemecek, R.; Svoboda, M.; Slaby, O. MicroRNAs targeting EGFR signalling pathway in colorectal cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Doi, K.; Fujimoto, T.; Tanaka, Y.; Ogawa, M.; Matsuzaki, H.; Kuroki, M.; Miyamoto, S.; Shirasawa, S.; Tsunoda, T. KRAS up-regulates the expression of miR-181a, miR-200c and miR-210 in a three-dimensional-specific manner in DLD-1 colorectal cancer cells. Anticancer Res. 2012, 32, 2271–2275. [Google Scholar] [PubMed]
- Cappuzzo, F.; Sacconi, A.; Landi, L.; Ludovini, V.; Biagioni, F.; D’Incecco, A.; Capodanno, A.; Salvini, J.; Corgna, E.; Cupini, S.; et al. MicroRNA signature in metastatic colorectal cancer patients treated with anti-EGFR monoclonal antibodies. Clin. Colorectal Cancer 2014, 13, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lupini, L.; Bassi, C.; Mlcochova, J.; Musa, G.; Russo, M.; Vychytilova-Faltejskova, P.; Svoboda, M.; Sabbioni, S.; Nemecek, R.; Slaby, O.; et al. Prediction of response to anti-EGFR antibody-based therapies by multigene sequencing in colorectal cancer patients. BMC Cancer 2015, 15, 808. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Winter, E.; Ress, A.L.; Bauernhofer, T.; Gerger, A.; Kiesslich, T.; Lax, S.; Samonigg, H.; Hoefler, G. miR-181a is associated with poor clinical outcome in patients with colorectal cancer treated with egfr inhibitor. J. Clin. Pathol. 2014, 67, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Kurihara, H.; Mitsuhashi, K.; Ito, M.; Okuda, H.; Kanno, S.; Naito, T.; Yoshii, S.; Takahashi, H.; Kusumi, T.; et al. Association of microRNA-31-5p with clinical efficacy of anti-EGFR therapy in patients with metastatic colorectal cancer. Ann. Surg. Oncol. 2015, 22, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Markman, J.L.; Shiao, S.L. Impact of the immune system and immunotherapy in colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 208–223. [Google Scholar] [PubMed]
- Li, X.; Nie, J.; Mei, Q.; Han, W.D. MicroRNAs: Novel immunotherapeutic targets in colorectal carcinoma. World J. Gastroenterol. 2016, 22, 5317–5331. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.E.; Simoes, A.E.; Pereira, D.M.; Castro, R.E.; Rodrigues, C.M.; Borralho, P.M. miR-143 or miR-145 overexpression increases cetuximab-mediated antibody-dependent cellular cytotoxicity in human colon cancer cells. Oncotarget 2016, 7, 9368–9387. [Google Scholar] [PubMed]
- Zhou, J.; Lv, L.; Lin, C.; Hu, G.; Guo, Y.; Wu, M.; Tian, B.; Li, X. Combinational treatment with microRNA133b and cetuximab has increased inhibitory effects on the growth and invasion of colorectal cancer cells by regulating egfr. Mol. Med. Rep. 2015, 12, 5407–5414. [Google Scholar] [PubMed]
- Toh, J.W.; de Souza, P.; Lim, S.H.; Singh, P.; Chua, W.; Ng, W.; Spring, K.J. The potential value of immunotherapy in colorectal cancers: Review of the evidence for programmed death-1 inhibitor therapy. Clin. Colorectal Cancer 2016, 15, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Kopetz, S.; McDermott, R.S.; Leach, J.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.D.; et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (msi-h): Checkmate-142 interim results. ASCO Meet. 2016. [Google Scholar] [CrossRef]
- Bendell, J.C.; Kim, T.W.; Goh, B.C.; Wallin, J.; Oh, D.-Y.; Han, S.-W.; Lee, C.B.; Hellmann, M.D.; Desai, J.; Lewin, J.H.; et al. Clinical activity and safety of cobimetinib and atezolizumab in colorectal cancer. ASCO Meet. 2016. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. Pd-l1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, H.; Yi, S.; Peng, X.; Su, P.; Xiao, Z.; Liu, R.; Tang, A.; Li, X.; Liu, F.; et al. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget 2016, 7, 45370–45384. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chen, D.; Lu, B.; Wang, C.; Zhang, J.; Huang, L.; Wang, X.; Timmons, C.L.; Hu, J.; Liu, B.; et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE 2013, 8, e65821. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, Y.; Farazmandfar, T.; Azadeh, H.; Zekavatian, Z. The prognostic effect of pten expression status in colorectal cancer development and evaluation of factors affecting it: miR-21 and promoter methylation. J. Biomed. Sci. 2016, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Nowak, J.A.; Kim, S.A.; Song, M.; Inamura, K.; Sukawa, Y.; Masuda, A.; Yang, J.; Dou, R.; et al. MicroRNA miR21 and t cells in colorectal cancer. Cancer Immunol. Res. 2016, 4, 33–40. [Google Scholar] [CrossRef] [PubMed]
| Prognostic ncRNA * | Tissue | Effect | Ref. |
|---|---|---|---|
| Conventional cytostatics | |||
| miR-21-5p | Tumor | Upregulation increases resistance to 5-FU | [7,8] |
| miR-129-5p | Tumor | Downregulation increases resistance to 5-FU | [10] |
| miR-429 | Tumor | Upregulation increases resistance to 5-FU | [11] |
| miR-577 | Tumor | Upregulation increases resistance to 5-FU | [12] |
| miR-1915-3p | Tumor | Downregulation increases resistance to chemotherapy | [13] |
| miR-122-5p | Tumor | Downregulation increases resistance to 5-FU | [14] |
| miR-21-5p | Tumor | Upregulation decreases resistance to nCRT | [16] |
| miR-153-3p | Tumor | Upregulation increases resistance to oxaliplatin | [18] |
| miR-19a-3p | Tumor | Upregulation increases resistance to FOLFOX therapy | [34] |
| miR-106a-5p, miR-130b-3p, miR-484 | Plasma/serum | Upregulation increases resistance to FOLFOX therapy | [21] |
| miR-20a-5p, miR-130, miR-145-5p, miR-216a-5p and miR-372-3p | Plasma/serum | Response prediction to 5-FU-based adjuvant therapy | [22] |
| TUG1 | Tumor | Upregulation increases resistance to methotrexate | [23] |
| MALAT1 | Tumor | Upregulation increases resistance to FOLFOX | [24] |
| UCA1 | Tumor | Upregulation increases resistance to 5-FU | [25] |
| Anti-VEGF antibodies | |||
| miR-126-3p | Plasma/serum | Upregulation increases resistance to CT and bevacizumab | [29] |
| miR-664-3p | Tumor | Upregulation increases sensitivity to CT and bevacizumab | [32] |
| miR-455-5p | Tumor | Downregulation increases sensitivity to CT and bevacizumab | [32] |
| Anti-EGFR antibodies | |||
| miR-99a-5p/let-7c/miR-125b-5p | Tumor | Response prediction to EGFR target-therapies | [37] |
| miR-31-3p and miR-31-5p | Tumor | Response prediction to cetuximab but not panitumumab | [38,39] |
| miR-31-5p | Tumor | Upregulation increases resistance to EGFR target-therapies | [40] |
| miR-181a-5p | Tumor | Downregulation increases resistance to EGFR target-therapies | [39] |
| miR-143-3p | Tumor | Upregulation increases sensitivity to cetuximab | [43] |
| miR-145-5p | Tumor | Upregulation increases sensitivity to cetuximab | [43] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garajová, I.; Ferracin, M.; Porcellini, E.; Palloni, A.; Abbati, F.; Biasco, G.; Brandi, G. Non-Coding RNAs as Predictive Biomarkers to Current Treatment in Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1547. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071547
Garajová I, Ferracin M, Porcellini E, Palloni A, Abbati F, Biasco G, Brandi G. Non-Coding RNAs as Predictive Biomarkers to Current Treatment in Metastatic Colorectal Cancer. International Journal of Molecular Sciences. 2017; 18(7):1547. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071547
Chicago/Turabian StyleGarajová, Ingrid, Manuela Ferracin, Elisa Porcellini, Andrea Palloni, Francesca Abbati, Guido Biasco, and Giovanni Brandi. 2017. "Non-Coding RNAs as Predictive Biomarkers to Current Treatment in Metastatic Colorectal Cancer" International Journal of Molecular Sciences 18, no. 7: 1547. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071547