Differential miR-346 and miR-582-3p Expression in Association with Selected Maternal and Fetal Complications
Abstract
:1 Introduction
2. Results
2.1. Clinical Characteristics of Included Groups and Sample Collection
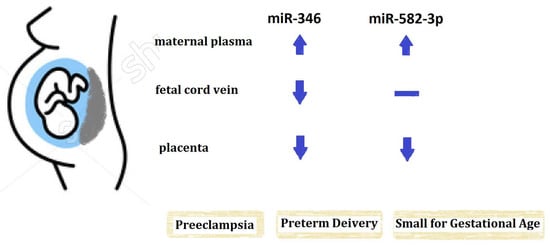
2.2. The Expression of miR-346 in Maternal Peripheral Blood, Fetal Cord Blood and Placenta
2.3. The Expression of miR-582-3p in Maternal Peripheral Blood, Fetal Cord Blood and Placenta
3. Discussion
4. Materials and Methods
4.1. Study Population and Sample Collection
4.2. miRNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, K.; Rajewsky, N. The evolution of gene regulation by transcription factors and microRNAs. Nat. Rev. Genet. 2007, 8, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. MicroRNomics: A newly emerging approach for disease biology. Physiol. Genom. 2008, 33, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, S.; Suarez, Y.; Skoura, A.; Offermanns, S.; Miano, J.M.; Sessa, W.C. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- D'angelo, B.; Benedetti, E.; Cimini, A.; Giordano, A. MicroRNAs: A puzzling tool in cancer diagnostics and therapy. Anticancer Res. 2016, 36, 5571–5575. [Google Scholar] [CrossRef] [PubMed]
- Meydan, C.; Shenhar-Tsarfaty, S.; Soreq, H. MicroRNA regulators of anxiety and metabolic disorders. Trends Mol. Med. 2016, 22, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Chim, S.S.; Shing, T.K.; Hung, E.C.; Leung, T.Y.; Lau, T.K.; Chiu, R.W.; Lo, Y.M. Detection and characterization of placental microRNAs in maternal plasma. Clin. Chem. 2008, 54, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Chaiwangyen, W.; Ospina-Prieto, S.; Schneider, U.; Herrmann, J.; Gruhn, B.; Markert, U.R. MicroRNA expression profiles of trophoblastic cells. Placenta 2012, 33, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Galliano, D.; Pellicer, A. MicroRNA and implantation. Fertil. Steril. 2014, 101, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, B.; Wang, J.; Lei, J.; Liu, C.; Ma, Y.; Zhao, H. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod. BioMed Online 2012, 25, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N. Uterine microRNA signature and consequence of their dysregulation in uterine disorders. Anim. Reprod. 2010, 7, 117–128. [Google Scholar] [PubMed]
- Morales Prieto, D.M.; Markert, U.R. MicroRNAs in pregnancy. J. Reprod. Immunol. 2011, 88, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, Q.; Warrick, J.; Lockwood, C.M.; Woodworth, A.; Moley, K.H.; Gronowski, A.M. Circulating microRNA miR-323–3p as a biomarker of ectopic pregnancy. Clin. Chem. 2012, 58, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Hasegawa, Y.; Abe, S.; Higashijima, A.; Miura, S.; Mishima, H.; Kinoshita, A.; Kaneuchi, M.; Yoshiura, K.; Masuzaki, H. Clinical applications of analysis of plasma circulating complete hydatidiform mole pregnancy-associated miRNAs in gestational trophoblastic neoplasia: A preliminary investigation. Placenta 2014, 35, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tian, F.; Li, H.; Zhou, Y.; Lu, J.; Ge, Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2015, 130, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Su, M.T.; Tsai, P.Y.; Tsai, H.L.; Chen, Y.C.; Kuo, P.L. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. BioFactors 2017, 43, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Catalano, R.D.; Morgan, K.; Critchley, H.O.D.; Millar, R.P.; Jabbour, H.N. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology 2008, 149, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Hoffmann, P.; Feige, J.J.; Alfaidy, N. EG-VEGF: A key endocrine factor in placental development. Trends Endocrinol. Metab. 2012, 23, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Murthi, P.; Hoffmann, P.; Salomon, A.; Sergent, F.; De Mazancourt, P.; Dakouane-Giudicelli, M.; Dieudonné, M.N.; Rozenberg, P.; Vaiman, D.; et al. EG-VEGF controls placental growth and survival in normal and pathological pregnancies: Case of fetal growth restriction (FGR). Cell Mol. Life Sci. 2013, 70, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Romero, R.; Kim, J.S.; Tarca, A.L.; Montenegro, D.; Pineles, B.L.; Kim, E.; Lee, J.; Kim, S.Y.; Draghici, S.; et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell Lines: Siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am. J. Pathol. 2011, 179, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Pineles, B.L.; Romero, R.; Montenegro, D.; Tarca, A.L.; Han, Y.M.; Kim, Y.M.; Draghici, S.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am. J. Obs. Gynecol. 2007, 196, 261.e1–261.e6. [Google Scholar] [CrossRef] [PubMed]
- Mouillet, J.F.; Chu, T.; Hubel, C.A.; Nelson, D.M.; Parks, W.T.; Sadovsky, Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta 2010, 31, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S. Cell Types of the Placenta. In Vascular Biology of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010; Chapter 4. [Google Scholar]
- Larrea, E.; Sole, C.; Manterola, L.; Goicoechea, I.; Armesto, M.; Arestin, M.; Caffarel, M.M.; Araujo, A.M.; Araiz, M.; Fernandez-Mercado, M.; et al. New Concepts in Cancer Biomarkers: Circulating miRNAs in Liquid Biopsies. Int. J. Mol. Sci. 2016, 17, 627. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental exosomes as early biomarker of preeclampsia—Potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, L.; Burlakov, J.; Hass, S.; von Kaisenberg, C.; von Versen-Höynck, F. Impaired functional capacity of fetal endothelial cells in preeclampsia. PLoS ONE 2017, 12, e0178340. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lau, S.Y.; Blenkiron, C.; Chen, Q.; James, J.L.; Kleffmann, T.; Wise, M.; Stone, P.R.; Chamley, L.W. Trophoblastic debris modifies endothelial cell transcriptome in vitro: A mechanism by which fetal cells might control maternal responses to pregnancy. Sci. Rep. 2016, 6, 30632. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hatt, L.; Ravn, K.; Vogel, I.; Petersen, O.B.; Uldbjerg, N.; Schelde, P. Fetal cells in maternal blood for prenatal diagnosis: A love story rekindled. Biomark. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- AbdelHalim, R.M.; Ramadan, D.I.; Zeyada, R.; Nasr, A.S.; Mandour, I.A. Circulating Maternal Total Cell-Free DNA, Cell-Free Fetal DNA and Soluble Endoglin Levels in Preeclampsia: Predictors of Adverse Fetal Outcome? A Cohort Study. Mol. Diagn. Ther. 2016, 20, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S. Placental Blood Circulation. In Vascular Biology of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010; Chapter 2. [Google Scholar]
- Fu, G.; Brkić, J.; Hayder, H.; Peng, C. MicroRNAs in human placental development and pregnancy complications. Int. J. Mol. Sci. 2013, 14, 5519. [Google Scholar] [CrossRef] [PubMed]
- Mouillet, J.F.; Chu, T.; Sadovsky, Y. Expression patterns of placental microRNAs. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 737–7843. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Doucha, J.; Dlouha, K.; Krofta, L. Absolute and relative quantification of placenta-specific microRNAs in maternal circulation with placental insufficiency—Related complications. J. Mol. Diagn. 2012, 14, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, A.; Miura, K.; Mishima, H.; Kinoshita, A.; Jo, O.; Abe, S.; Hasegawa, Y.; Miura, S.; Yamasaki, K.; Yoshida, A.; et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat. Diagn. 2013, 33, 214–2222. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Ma, N.; Xu, Y.; Jiang, L.; Yang, J.; Wang, C.; Jiao, Y.; Gao, X. Differential distribution of U6 (RNU6–1) expression in human carcinoma tissues demonstrates the requirement for caution in the internal control gene selection for microRNA quantification. Int. J. Mol. Med. 2015, 36, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Zeng, Y.; Yang, R.; Xu, H.; Chen, Z.; Zhong, J.; Xie, H.; Xu, Y.; Zeng, X. U6 is not a suitable endogenous control for the quantification of circulating microRNAs. Biochem. Biophys. Res. Commun. 2014, 454, 210–214. [Google Scholar] [CrossRef] [PubMed]


| Clinical Information | Normal Control (n = 60) | Preeclampsia (n = 31) | Preterm Delivery (n = 29) | Small for Gestational Age (n = 19) |
|---|---|---|---|---|
| Maternal Age (y) | 31.33 ± 4.31 | 33.83 ± 5.77 | 32.04 ± 5.14 | 31.68 ± 7.21 |
| Nulliparity (%) | 56.7% | 48.4% | 34.5% * | 73.7% |
| Gestational Age at Delivery (week) | 38.87 ± 0.99 | 36.84 ± 2.30 * | 34.73 ± 2.00 * | 36.87 ± 3.01 * |
| Ethnicity (Chinese Hans %) | 100% | 100% | 96.3% | 89.5% |
| Cesarean Section | 35.0% | 80.7% * | 51.9% | 47.4% |
| BMI at Delivery (kg/m2) | 26.47 ± 3.55 | 29.10 ± 5.60 | 26.04 ± 4.46 | 24.67 ± 3.16 |
| Neonatal Outcome | ||||
| Birth Weight (g) | 3192 ± 299.59 | 2622 ± 676.74 * | 2214 ± 502.45 * | 2173 ± 598.61 * |
| Sex (female %) | 50% | 50% | 51.7% | 57.9% |
| 1 min Apgar Score | 8.635 ± 0.71 | 8.063 ± 1.16 * | 7.36 ± 1.04 * | 7.938 ± 1.18 |
| 5 min Apgar Score | 9.827 ± 0.43 | 9.469 ± 0.88 | 9.160 ± 0.75 * | 9.438 ± 0.89 |
| Placenta Weight (g) | 631.4 ± 113.95 | 560 ± 158.32 * | 562.1 ± 182.86 * | 437.4 ± 113.32 * |
| Specimen Origin | Normal Control (n = 60) | Preeclampsia (n = 31) | Preterm Deliver (n = 29) | Small for Gestational Age (n = 19) |
|---|---|---|---|---|
| miR-346 | ||||
| Maternal Plasma | 0.12 (0.02, 0.36) | 0.51 * (0.07, 1.00) | 2.11 * (0.63, 6.88) | 0.62 * (0.13, 1.56) |
| Fetal Cord Plasma | 23.98 (4.37, 144.31) | 9.74 * (1.91, 21.89) | 7.21 (1.23, 44.09) | 5.96 (1.00, 25.00) |
| Placenta | 1.00 (0.61, 2.06) | 0.54 * (0.27, 0.81) | 0.18 * (0.11, 1.00) | 0.56 * (0.26, 1.02) |
| miR-582-3p | ||||
| Maternal Plasma | 5.64 × 10−5 (0.07 × 10−5, 24.76 × 10−5) | 4.17 × 10−3 * (0.20 × 10−3, 16.31 × 10−3) | 6.09 × 10−2 * (0.08 × 10−2, 29.50 × 10−2) | 5.48 × 10−3 (0.02 × 10−1, 37.01 × 10−1) |
| Fetal Cord Plasma | 1.67 × 10−3 (0.05 × 10−3, 56.60 × 10−3) | 1.14 × 10−2 (0.01 × 10−2, 6.72 × 10−2) | 9.28 × 10−3 (0.24 × 10−3, 469.52 × 10−3) | 1.02 × 10−3 (0.02 × 10−3, 17.39 × 10−3) |
| Placenta | 1.56 (0.83, 2.45) | 0.75 (0.39, 11.37) | 0.23 * (0.14, 0.80) | 0.70 * (0.28, 0.95) |
| Specimen Origin | Normal Control (n = 60) | Preeclampsia (n = 31) | Preterm Delivery (n = 29) | Small for Gestational Age (n = 19) | |||
|---|---|---|---|---|---|---|---|
| slope | p value | slope | p value | slope | p value | ||
| miR-346 | |||||||
| Log Maternal plasma | 1 | 1.13 * | 0.049 | 2.50 * | <0.001 | 1.16 | 0.068 |
| Log Fetal cord plasma | 1 | −1.45 * | 0.008 | −1.81 * | 0.006 | −1.50 * | 0.015 |
| Log Placenta | 1 | −0.61 | 0.059 | −1.53 * | <0.001 | −0.99 * | 0.007 |
| miR-582-3p | |||||||
| Log Maternal plasma | 1 | 3.14 * | 0.020 | 4.20 * | 0.010 | 2.44 | 0.099 |
| Log Fetal cord plasma | 1 | 1.37 | 0.455 | 2.18 | 0.185 | −0.36 | 0.812 |
| Log Placenta | 1 | 0.21 | 0.603 | −1.67* | <0.001 | −1.14 * | 0.012 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, P.-Y.; Li, S.-H.; Chen, W.-N.; Tsai, H.-L.; Su, M.-T. Differential miR-346 and miR-582-3p Expression in Association with Selected Maternal and Fetal Complications. Int. J. Mol. Sci. 2017, 18, 1570. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071570
Tsai P-Y, Li S-H, Chen W-N, Tsai H-L, Su M-T. Differential miR-346 and miR-582-3p Expression in Association with Selected Maternal and Fetal Complications. International Journal of Molecular Sciences. 2017; 18(7):1570. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071570
Chicago/Turabian StyleTsai, Pei-Yin, Sheng-Hsiang Li, Wan-Ni Chen, Hui-Ling Tsai, and Mei-Tsz Su. 2017. "Differential miR-346 and miR-582-3p Expression in Association with Selected Maternal and Fetal Complications" International Journal of Molecular Sciences 18, no. 7: 1570. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071570