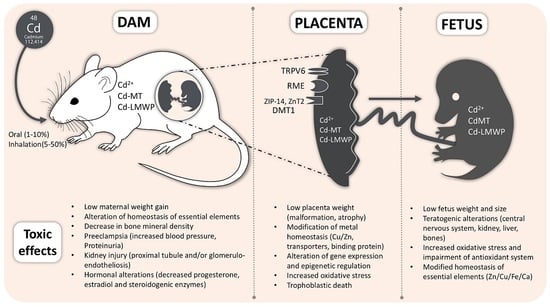
Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models
Abstract
:1. Introduction
2. General Information about Cd
2.1. Mode of Action (MOA) of Cd Toxicity
2.2. Sources of Exposure
3. Cadmium Distribution during Pregnancy
3.1. Role of Diet
3.2. The Role of DMT-1 in Cd Distribution during Pregnancy
3.3. Role of Metallothionein in Cd Distribution
4. Toxic Effects in Dams
4.1. Preeclampsia
4.2. Kidney Damage
4.3. Lesser Incidence of Pregnancy
4.4. Altered Levels of Essential Elements in the Body
4.5. Decreased Bone Mineral Density
5. Transport of Cd across the Placenta
5.1. Placental Susceptibility to Cd
5.2. Cadmium Distribution in the Fetus
6. Effects of Cd in the Fetal Organism
6.1. Teratogenicity
6.2. Central Nervous System
6.3. Liver
6.4. Kidney
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kjellstrom, T.; Nordberg, G.F. A kinetic model of cadmium metabolism in the human being. Environ. Res. 1978, 16, 248–269. [Google Scholar] [CrossRef]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. U.S.Toxicological Profile for Cadmium; Department of Health and Human Sevices, Public Health Service, Centers for Disease Control Atlanta; ATSDR: Atlanta, GA, USA, 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15 (accessed on 29 June 2017).
- Goyer, R.; Clarkson, T. Toxics effects of metals. In Casarett and Doull's Toxicology: The Basic Science of Poisons; Klaassen, C.D., Ed.; McGraw-Hill, Health Professions Division: New York, NY, USA, 2013. [Google Scholar]
- Rogers, J. Developmental toxicology. In Casarett and Doull's Toxicology: The Basic Science of Poisons; Klaassen, C.D., Ed.; McGraw-Hill, Health Professions Division: New York, NY, USA, 2013. [Google Scholar]
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Neesen, J.; Straka, E.; Ellinger, I.; Dolznig, H.; Hengstschlager, M. Genetics of the human placenta: Implications for toxicokinetics. Arch. Toxicol. 2016, 90, 2563–2581. [Google Scholar] [CrossRef] [PubMed]
- Little, M.H. Improving our resolution of kidney morphogenesis across time and space. Curr. Opin. Genet. Dev. 2015, 32, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Patro-Malysza, J.; Kimber-Trojnar, Z.; Marciniak, B.; Oleszczuk, J.; Leszczynska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Rabah, A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int. J. Hyg. Environ. Health 2014, 217, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Wilson, R.E. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum. Dev. 2007, 83, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; McCullough, L.E.; Tzeng, J.Y.; Darrah, T.; Vengosh, A.; Maguire, R.L.; Maity, A.; Samuel-Hodge, C.; Murphy, S.K.; Mendez, M.A.; et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health 2017, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.P.; Claus Henn, B.; Wright, R.O. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: A review of recent literature. Curr. Environ. Health Rep. 2015, 2, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Appleton, A.; Jackson, B.; Karagas, M.; Marsit, C. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 2017. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Vahter, M.; Broberg, K. The epigenetic effects of prenatal cadmium exposure. Curr. Environ. Health Rep. 2015, 2, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Jacquillet, G.; Tauc, M.; Cougnon, M.; Poujeol, P. Effect of heavy metals on, and handling by, the kidney. Nephron Physiol. 2005, 99, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F. Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 2010, 23, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Lee, W.K. Toxicology of cadmium and its damage to mammalian organs. Met. Ions Life Sci. 2013, 11, 415–490. [Google Scholar] [PubMed]
- Smith, J.B.; Dwyer, S.D.; Smith, L. Cadmium evokes inositol polyphosphate formation and calcium mobilization. Evidence for a cell surface receptor that cadmium stimulates and zinc antagonizes. J. Biol. Chem. 1989, 264, 7115–7118. [Google Scholar] [PubMed]
- Yang, H.; Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int. J. Mol. Sci. 2015, 16, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F. Cadmium and cellular signaling cascades: To be or not to be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Apaijit, K.; Kukongviriyapan, V. Oxidative stress and cardiovascular dysfunction associated with cadmium exposure: Beneficial effects of curcumin and tetrahydrocurcumin. Tohoku J. Exp. Med. 2016, 239, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Fassett, D. Metallic Contaminants and Human Health; Academic Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Cadmium. Background and National Experience with Reducing Risk; Organisation for Economic Co-Operation and Development: Paris, France, 1995; Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?doclanguage=en&cote=ocde/gd(94)97 (accessed on 29 June 2017).
- Emsley, J. Nature’s Building Blocks: An a-z Guide to the Elements; Oxford University Press: Oxford, UK, 2011; Volume 48, p. 22. [Google Scholar]
- Kosanovic, M.; Jokanovic, M.; Jevremovic, M.; Dobric, S.; Bokonjic, D. Maternal and fetal cadmium and selenium status in normotensive and hypertensive pregnancy. Biol. Trace Elem. Res. 2002, 89, 97–103. [Google Scholar] [CrossRef]
- Jarup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24, 1–51. [Google Scholar] [PubMed]
- Saldivar, L.; Luna, M.; Reyes, E.; Soto, R.; Fortoul, T.I. Cadmium determination in mexican-produced tobacco. Environ. Res. 1991, 55, 91–96. [Google Scholar] [CrossRef]
- Elinder, C.G.; Kjellstrom, T.; Lind, B.; Linnman, L.; Piscator, M.; Sundstedt, K. Cadmium exposure from smoking cigarettes: Variations with time and country where purchased. Environ. Res. 1983, 32, 220–227. [Google Scholar] [CrossRef]
- Rebelo, F.M.; Caldas, E.D. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environ. Res. 2016, 151, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Jacquillet, G.; Barbier, O.; Rubera, I.; Tauc, M.; Borderie, A.; Namorado, M.C.; Martin, D.; Sierra, G.; Reyes, J.L.; Poujeol, P.; et al. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am. J. Physiol. Ren. Physiol. 2007, 293, F1450–F1460. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.L.; Edwards, J.R.; Prozialeck, W.C.; Xiong, J.Q.; Zelikoff, J.T. Effects of maternal exposure to cadmium oxide nanoparticles during pregnancy on maternal and offspring kidney injury markers using a murine model. J. Toxicol. Environ. Health 2015, 78, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Edgerton, S.A.; Vega, E. Chemical composition of PM2.5 and PM10 in mexico city during winter 1997. Sci. Total Environ. 2002, 287, 177–201. [Google Scholar] [CrossRef]
- Guerra, R.; Vera-Aguilar, E.; Uribe-Ramirez, M.; Gookin, G.; Camacho, J.; Osornio-Vargas, A.R.; Mugica-Alvarez, V.; Angulo-Olais, R.; Campbell, A.; Froines, J.; et al. Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicol. Lett. 2013, 222, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Leazer, T.M.; Liu, Y.; Klaassen, C.D. Cadmium absorption and its relationship to divalent metal transporter-1 in the pregnant rat. Toxicol. Appl. Pharmacol. 2002, 185, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mikolić, A.; Schonwald, N.; Piasek, M. Cadmium, iron and zinc interaction and hematological parameters in rat dams and their offspring. J. Trace Elem. Med. Biol. 2016, 38, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Phillips, L.; Sanford, J.; Wooton, M.; Gregg, A.; Schuda, L. A review of physiological and behavioral changes during pregnancy and lactation: Potential exposure factors and data gaps. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Astbury, S.; Mostyn, A.; Symonds, M.E.; Bell, R.C. Nutrient availability, the microbiome, and intestinal transport during pregnancy. Appl. Physiol. Nutr. Metab. 2015, 40, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Estrada, T.; Cardenas-Gonzalez, M.; Santoyo-Sanchez, M.; Parada-Cruz, B.; Uria-Galicia, E.; Arreola-Mendoza, L.; Barbier, O. Evaluation of kidney injury biomarkers in rat amniotic fluid after gestational exposure to cadmium. J. Appl. Toxicol. 2016, 36, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohba, K.; Ohta, H. Participation of metal transporters in cadmium transport from mother rat to fetus. J. Toxicol. Sci. 2012, 37, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Mikolić, A.; Piasek, M.; Sulimanec Grgec, A.; Varnai, V.M.; Stasenko, S.; Kralik Oguić, S. Oral cadmium exposure during rat pregnancy: Assessment of transplacental micronutrient transport and steroidogenesis at term. J. Toxicol. Sci. 2015, 35, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.L.; Xiong, J.Q.; Hoffman, C.; Zelikoff, J.T. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol. Sci. 2012, 126, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Petersson Grawe, K.; Oskarsson, A. Cadmium in milk and mammary gland in rats and mice. Arch. Toxicol. 2000, 73, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Brako, E.E.; Wilson, A.K.; Jonah, M.M.; Blum, C.A.; Cerny, E.A.; Williams, K.L.; Bhattacharyya, M.H. Cadmium pathways during gestation and lactation in control versus metallothoinein 1,2-knockout mice. Toxicol. Sci. 2003, 71, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Vesey, D.A. Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Lett. 2010, 198, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Ueda, H.; Tanaka, K. Involvement of intestinal calcium transporter 1 and metallothionein in cadmium accumulation in the liver and kidney of mice fed a low-calcium diet. Toxicol. Lett. 2008, 176, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Sano, E.; Ueda, H.; Sakazaki, F.; Yamada, K.; Takano, M.; Tanaka, K. Dietary deficiency of calcium and/or iron, an age-related risk factor for renal accumulation of cadmium in mice. Biol. Pharm. Bull. 2015, 38, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Momoi, K.; Hosoyamada, M.; Kimura, M.; Shibasaki, T. Normal cadmium uptake in microcytic anemia mk/mk mice suggests that DMT1 is not the only cadmium transporter in vivo. Toxicol. Appl. Pharmacol. 2008, 227, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Jacquillet, G.; Barbier, O.; Cougnon, M.; Tauc, M.; Namorado, M.C.; Martin, D.; Reyes, J.L.; Poujeol, P. Zinc protects renal function during cadmium intoxication in the rat. Am. J. Physiol. Ren. Physiol. 2006, 290, F127–F137. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants-curcumin, resveratrol and melatonin- in cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 2015, 7, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.C. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 2015, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Ohrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.Y.; Choi, B.S.; Youn, P.; Ryu, D.Y.; Klaassen, C.D.; Park, J.D. Regulation of metal transporters by dietary iron, and the relationship between body iron levels and cadmium uptake. Arch. Toxicol. 2007, 81, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Liu, S.Y.; Wang, H.J.; Zhang, T.W.; Yu, P.; Duan, X.L.; Zhao, S.E.; Chang, Y.Z. Effects of pregnancy and lactation on iron metabolism in rats. BioMed Res. Int. 2015, 2015, 105325. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Shawki, A.; Ganz, T.; Nemeth, E.; Mackenzie, B. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am. J. Physiol. Cell Physiol. 2014, 306, C450–C459. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Choudhuri, S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.M.; Cherian, M.G. Mobilization of hepatic cadmium in pregnant rats. Toxicol. Appl. Pharmacol. 1993, 120, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Storm, T.; Christensen, E.I.; Christensen, J.N.; Kjaergaard, T.; Uldbjerg, N.; Larsen, A.; Honore, B.; Madsen, M. Megalin is predominantly observed in vesicular structures in first and third trimester cytotrophoblasts of the human placenta. J. Histochem. Cytochem. 2016, 64, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Klassen, R.B.; Crenshaw, K.; Kozyraki, R.; Verroust, P.J.; Tio, L.; Atrian, S.; Allen, P.L.; Hammond, T.G. Megalin mediates renal uptake of heavy metal metallothionein complexes. Am. J. Physiol. Ren. Physiol. 2004, 287, F393–F403. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.A.; Abouhamed, M.; Verroust, P.J.; Thevenod, F. Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured renal proximal tubule cells. J. Pharmacol. Exp. Ther. 2006, 318, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Onodera, A.; Tani, M.; Michigami, T.; Yamagata, M.; Min, K.S.; Tanaka, K.; Nakanishi, T.; Kimura, T.; Itoh, N. Role of megalin and the soluble form of its ligand RAP in Cd-metallothionein endocytosis and Cd-metallothionein-induced nephrotoxicity in vivo. Toxicol. Lett. 2012, 212, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Wolff, N.A. Iron transport in the kidney: Implications for physiology and cadmium nephrotoxicity. Metallomics 2016, 8, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Thevenod, F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS ONE 2013, 8, e71586. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.B.; Stanley, J.A.; Princess, R.A.; Shanthi, P.; Sebastian, M.S. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J. Med. Toxicol. 2011, 7, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Z.; Lin, F.; Wang, F.; Ye, D.; Huang, Y. Increased oxidative DNA damage in placenta contributes to cadmium-induced preeclamptic conditions in rat. Biol. Trace Elem. Res. 2016, 170, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Q.; Zhang, X.; Luo, S.; Ye, D.; Guo, Y.; Chen, S.; Huang, Y. Preeclampsia induced by cadmium in rats is related to abnormal local glucocorticoid synthesis in placenta. Reprod. Biol. Endocrinol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Zhang, K.; Huang, Y.; Yan, Y.; Wang, F.; Wu, J.; Wang, X.; Xu, Z.; Chen, Y.; et al. Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ. Pollut. 2016, 218, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.M.; Urrutia, M.; Montenegro, M.; Llanos, M.N. Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicol. Lett. 2009, 188, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, L.P.; Gupta, S. Biochemical effects of gestational coexposure to lead and cadmium on reproductive performance, placenta, and ovary. J. Biochem. Mol. Toxicol. 2008, 22, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Lee, J.T.; Yu, S.J.; Kang, S.G.; Moon, C.S.; Choi, Y.H.; Kim, J.H.; Kim, D.H.; Son, B.C.; Lee, C.H.; et al. Effects of cadmium on the expression of placental lactogens and pit-1 genes in the rat placental trophoblast cells. Mol. Cell. Endocrinol. 2009, 298, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Piasek, M.; Laskey, J.W. Acute cadmium exposure and ovarian steroidogenesis in cycling and pregnant rats. Reprod. Toxicol. 1994, 8, 495–507. [Google Scholar] [CrossRef]
- Chmielnicka, J.; Sowa, B. Cadmium interaction with essential metals (Zn, Cu, Fe), metabolism metallothionein, and ceruloplasmin in pregnant rats and fetuses. Ecotoxicol. Environ. Saf. 1996, 35, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Trottier, B.; Athot, J.; Ricard, A.C.; Lafond, J. Maternal-fetal distribution of cadmium in the guinea pig following a low dose inhalation exposure. Toxicol. Lett. 2002, 129, 189–197. [Google Scholar] [CrossRef]
- Wang, C.; Brown, S.; Bhattacharyya, M.H. Effect of cadmium on bone calcium and 45Ca in mouse dams on a calcium-deficient diet: Evidence of itai-itai-like syndrome. Toxicol. Appl. Pharmacol. 1994, 127, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Otha, H.; Ichikawa, M.; Seki, Y. Effects of cadmium intake on bone metabolism of mothers during pregnancy and lactation. Tohoku J. Exp. Med. 2002, 196, 33–42. [Google Scholar]
- Pauli, J.M.; Repke, J.T. Preeclampsia: Short-term and long-term implications. Obstet. Gynecol. Clin. N. Am. 2015, 42, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Redecha, P.B.; Burmeister, M.A.; Tomlinson, S.; D'Agati, V.D.; Davisson, R.L.; Salmon, J.E. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011, 79, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Chamorro-Cevallos, G. Glycine reduces cadmium-induced teratogenic damage in mice. Reprod. Toxicol. 2007, 23, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, L.; Kuang, H.; Yang, P.; Aguilar, Z.P.; Wang, A.; Fu, F.; Xu, H. Acute toxicity of quantum dots on late pregnancy mice: Effects of nanoscale size and surface coating. J. Hazard. Mater. 2016, 318, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hofer, N.; Diel, P.; Wittsiepe, J.; Wilhelm, M.; Degen, G.H. Dose- and route-dependent hormonal activity of the metalloestrogen cadmium in the rat uterus. Toxicol. Lett. 2009, 191, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Yarilin, D.; Thurman, J.M.; Holers, V.M.; Salmon, J.E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006, 203, 2165–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, A.M.; Salmon, J.E. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 2010, 31, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Denny, K.J.; Coulthard, L.G.; Finnell, R.H.; Callaway, L.K.; Taylor, S.M.; Woodruff, T.M. Elevated complement factor C5a in maternal and umbilical cord plasma in preeclampsia. J. Reprod. Immunol. 2013, 97, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Piasek, M.; Blanusa, M.; Kostial, K.; Laskey, J.W. Low iron diet and parenteral cadmium exposure in pregnant rats: The effects on trace elements and fetal viability. Biometals 2004, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C.; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. Copper transport. Am. J. Clin. Nutr. 1998, 67, 965S–971S. [Google Scholar] [PubMed]
- Lee, S.H.; Lancey, R.; Montaser, A.; Madani, N.; Linder, M.C. Ceruloplasmin and copper transport during the latter part of gestation in the rat. Proc. Soc. Exp. Biol. Med. 1993, 203, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.L.; Sauble, E.N.; Cabrera, A.; Roth, A.; Ackland, M.L.; Mercer, J.F.; Linder, M.C. Lack of ceruloplasmin expression alters aspects of copper transport to the fetus and newborn, as determined in mice. Biometals 2012, 25, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohba, K.; Suzuki, K.; Ohta, H. Health effects of low-level cadmium intake and the role of metallothionein on cadmium transport from mother rats to fetus. J. Toxicol. Sci. 2012, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Daoud, G.; Bernatchez, R.; Simoneau, L.; Masse, A.; Lafond, J. Calcium uptake and calcium transporter expression by trophoblast cells from human term placenta. Biochim. Biophys. Acta 2002, 1564, 325–332. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kovacs, C.S.; Takanaga, H.; Peng, J.B.; Landowski, C.P.; Hediger, M.A. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J. Bone Miner. Res. 2008, 23, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Danko, T.; Bergeron, M.J.; Balazs, B.; Suzuki, Y.; Zsembery, A.; Hediger, M.A. Heavy metal cations permeate the TRPV6 epithelial cation channel. Cell Calcium 2011, 49, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Montalbetti, N.; Franz, M.C.; Graeter, S.; Simonin, A.; Hediger, M.A. Human TRPV5 and TRPV6: Key players in cadmium and zinc toxicity. Cell Calcium 2013, 54, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Olson, G.E.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Kurokawa, S. Maternal-fetal transfer of selenium in the mouse. FASEB J. 2013, 27, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Pařízek, J. Vascular changes at sites of oestrogen biosynthesis produced by parenteral injection of cadmium salts: The destruction of the placenta by cadmium salts. J. Reprod. Fertil. 1964, 7, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Pařízek, J. The peculiar toxicity of cadmium during pregnancy–an experimental “toxaemia of pregnancy“ induced by cadmium salts. J. Reprod. Fertil. 1965, 9, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.A.; Miller, R.K. Fetal toxicity of cadmium in the rat: Decreased utero-placental blood flow. Toxicol. Appl. Pharmacol. 1981, 58, 297–306. [Google Scholar] [CrossRef]
- Zhang, G.B.; Wang, H.; Hu, J.; Guo, M.Y.; Wang, Y.; Zhou, Y.; Yu, Z.; Fu, L.; Chen, Y.H.; Xu, D.X. Cadmium-induced neural tube defects and fetal growth restriction: Association with disturbance of placental folate transport. Toxicol. Appl. Pharmacol. 2016, 306, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Xu, Z.M.; Ji, Y.L.; Chen, Y.H.; Zhang, Z.H.; Zhang, C.; Meng, X.H.; Zhao, M.; Xu, D.X. Cadmium-induced teratogenicity: Association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol. Appl. Pharmacol. 2012, 259, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Furukawa, S.; Tanaka, A.; Kobayashi, Y.; Sugiyama, A. Histological localization of cadmium in rat placenta by LA-ICP-MS. J. Toxicol. Pathol. 2016, 29, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, Z.; Xi, Y.; Wang, L. Epigenetic regulation of placental glucose transporters mediates maternal cadmium-induced fetal growth restriction. Toxicology 2016, 372, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, L.; Hu, S.; Zhou, S.; Deng, Y.; Dong, M.; Huang, J.; Zeng, Y.; Chen, X.; Zhao, N.; et al. Down-regulation of ABCG2 and ABCB4 transporters in the placenta of rats exposed to cadmium. Oncotarget 2016, 7, 38154–38163. [Google Scholar] [CrossRef] [PubMed]
- Enli, Y.; Turgut, S.; Oztekin, O.; Demir, S.; Enli, H.; Turgut, G. Cadmium intoxication of pregnant rats and fetuses: Interactions of copper supplementation. Arch. Med. Res. 2010, 41, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Gupta, S. Effect of gestational and lactational exposure to lead and/or cadmium on reproductive performance and hepatic oestradiol metabolising enzymes. Toxicol. Lett. 2005, 155, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.; Cain, K. Functions of metallothionein. Biochem. Pharmacol. 1982, 31, 137–142. [Google Scholar] [CrossRef]
- Robinson, J.F.; Yu, X.; Hong, S.; Griffith, W.C.; Beyer, R.; Kim, E.; Faustman, E.M. Cadmium-induced differential toxicogenomic response in resistant and sensitive mouse strains undergoing neurulation. Toxicol. Sci. 2009, 107, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Couto-Moraes, R.; Felicio, L.F.; Bernardi, M.M. Post-partum testosterone administration does not reverse the effects of perinatal exposure to cadmium on rat offspring development. J. Appl. Toxicol. 2010, 30, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Payan, J.P.; Brondeau, M.T.; Zissu, D.; de Ceaurriz, J. Changes in urinary proximal tubule parameters in neonatal rats exposed to cadmium chloride during pregnancy. J. Appl. Toxicol. 1991, 11, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.; Ibanez, F.; Guajardo, A.; Llanos, M.N.; Ronco, A.M. Impact of cadmium exposure during pregnancy on hepatic glucocorticoid receptor methylation and expression in rat fetus. PLoS ONE 2012, 7, e44139. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.d.C.; González, N.; Gómez, S.; Quiroga, M.; Najle, R.; Barbeito, C. Effect of a single dose of cadmium on pregnant wistar rats and their offspring. Reprod. Domest. Anim. 2014, 49, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lee, G.S.; Shimizu, H.; Collins, M.D. Comparative molecular pathology of cadmium- and all-trans-retinoic acid-induced postaxial forelimb ectrodactyly. Toxicol. Appl. Pharmacol. 2007, 225, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Madrigal-Bujaidar, E.; Martinez-Galero, E.; Chamorro-Cevallos, G. Protection against cadmium-induced teratogenicity in vitro by glycine. Toxicol. In Vitro 2008, 22, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, F.; Talassi, C.B.; Salzgeber, S.A.; Spinosa, H.S.; Bernardi, M.M. Embryotoxic and long-term effects of cadmium exposure during embryogenesis in rats. Neurotoxicol. Teratol. 2004, 26, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J., Jr.; Schreiner, C.M.; Goetz, J.A.; Robbins, D.; Bell, S.M. Cadmium-induced postaxial forelimb ectrodactyly: Association with altered sonic hedgehog signaling. Reprod. Toxicol. 2005, 19, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Antonio Garcia, T.; Corredor, L. Biochemical changes in the kidneys after perinatal intoxication with lead and/or cadmium and their antagonistic effects when coadministered. Ecotoxicol. Environ. Saf. 2004, 57, 184–189. [Google Scholar] [CrossRef]
- Kuriwaki, J.; Nishijo, M.; Honda, R.; Tawara, K.; Nakagawa, H.; Hori, E.; Nishijo, H. Effects of cadmium exposure during pregnancy on trace elements in fetal rat liver and kidney. Toxicol. Lett. 2005, 156, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Kamihira, O.; Ozawa, H. Neural tube defects: Prevalence, etiology and prevention. Int. J. Urol. 2009, 16, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Oĝuz, E.O.; Abban, G.; Kutlubay, R.; Turgut, S.; Enli, Y.; Erdogan, D. Transmission electron microscopy study of the effects of cadmium and copper on fetal rat liver tissue. Biol. Trace Elem. Res. 2007, 115, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Roman, T.R.N.; De Lima, E.G.; Azoubel, R.; Batigália, F. Renal morphometry of fetuses rats treated with cadmium. Int. J. Morphol. 2004, 22, 231–236. [Google Scholar] [CrossRef]
- Chong, E.; Yosypiv, I.V. Developmental programming of hypertension and kidney disease. Int. J. Nephrol. 2012, 2012, 760580. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.K.; Thevenod, F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed]

| Outcome | Reference |
|---|---|
| Lower maternal weight gain. | [45,71] |
| Increase of systolic blood pressure and increased proteinuria associated with preeclampsia. | [72,73,74] |
| Abnormal glucocorticoid synthesis; induction of angiotensin II type 1-receptor-agonist autoantibodies (AT1-AA) and activation of complement component 5 (C5). | [73,74,75] |
| Kidney injury reported by excretion of Kim-1 into urine; increase of blood urea nitrogen; changes in the morphology of proximal tubules, like irregularly shaped nuclei and presence of vacuoles. Glomeruloendotheliosis and infiltrated inflammatory cells. | [2,29,52] |
| Decrease of progesterone and estradiol in placenta and plasma and decreased steroidogenic enzymes in reproductive organs. Increased uterine weight. | [45,76,77,78] |
| Alteration in the levels of essential elements in the body. | [39,43,44,79,80] |
| Decreased bone mineral density. | [81,82] |
| Outcome | Reference |
|---|---|
| Reduction of placental weight. | [77,87,105] |
| Decreased levels of total proteins, RNA, total lipids, cholesterol and glycogen. | [76] |
| Altered mRNA and protein levels, as well as activity of steroidogenic enzymes: Decreased: 3β-Hydroxysteroid dehydrogenase (HSD), 17β-HSD and 11β-HSD2. Increased: CYP11A1, CYP11B1 and CYP21. | [73,76] |
| Altered production of hormones necessary for the maintenance of pregnancy. | [76,77,87] |
| Placental atrophy, and swelling, vacuolization, deformation and death of trophoblasts due to apoptosis and necrosis in the junctional and labyrinthine zones of the placenta. Increased mRNA levels of molecules involved in apoptosis (p.53 and Bax). | [77,87,96,106,107] |
| DNA fragmentation in the junctional zone. | [77] |
| Decreased Zn and Cu placental concentration and altered metal transporters expression. | [38,43,87,96] |
| Altered MT mRNA and protein expression. | [43,107] |
| Oxidative stress and endoplasmic reticulum stress. | [87,106] |
| Reduced inner space of maternal and fetal blood vessels in the labyrinth layer. Thickening in the media vessel walls. | [73,105,106] |
| Higher corticosterone levels. | [73,75] |
| Decreased mRNA and protein levels of glucose transporter 3 (GLUT3). Hypermethylation of GLUT3 promoter region (E19.5). | [108] |
| Increased placental mRNA and protein levels of DNA methyltransferase 3-like (DNMT3L) and DNA methyltransferase 3 β (DNMT3B). | [108] |
| Lower protein levels of ATP-binding cassette (ABC) ABCG2 and ABCB4 transporters. | [109] |
| Decreased mRNA and protein levels of placental proton-coupled folate transporter (PCFT). | [105] |
| Outcome | Reference |
|---|---|
| Lower fetal body weight, length and head diameter. | [42,73,75,77,87,105,106,108,110,113,114,115,116] |
| Higher number of resorptions, dead fetuses and post-implantation losses. | [77,86,87,105,107,113,117] |
| Increased apoptosis and decreased proliferation in the mesenchyme of limb buds of embryos. | [118] |
| Embryos with lower morphological score, somites number, DNA content, yolk sac diameter and cephalic length. | [119] |
| Higher percentage of embryos with an open neural tube and altered expression of genes related to the development of the Central Nervous System (CNS) and cell cycle arrest. | [113,119] |
| Increased levels of malondialdehyde (MDA) and myeloperoxidase (MPO) and decreased levels of Glutathione (GSH), Superoxide dismutase (SOD) and catalase (CAT) in embryos, placenta, fetal kidneys (except MPO), and fetal liver. | [86,110,119] |
| Fetal symmetrical kidneys, and renal cavitation and damage (tubular necrosis and degeneration, presence of hyaline cylinders in tubules, and proteinaceous material in the renal pelvis). | [42,120] |
| Delayed chondrogenesis that leads to decreased ossification of head bones, sternebrae and cervical vertebrae. Further, Cd causes fused ribs and vertebrae. Absence or lesser number of vertebrae, skull bones, ribs, tail bones, metacarpal and metatarsal bones, and phalanges. | [86,117,118] |
| Increased frequency of abnormalities such as cleft palate, unilateral anophtalmia, microphtalmia, hypoplasic lungs, genital anomalies, vein deformation, postaxial forelimb ectrodactyly (predominantly right-sided and with the loss of digit 5), clubfoot, polydactyly, anencephaly, exencephaly, encephalomeningocele, micrognathia, exophthalmos, tail deformity, amelia, brachygnathia, omphalocele, anotia, hemoperitoneum, brain edema and undifferentiated limbs. | [86,87,105,106,113,117,118,120,121] |
| Higher Cd levels in blood, liver and kidneys in the offspring at postnatal days (PND) 0 through 60. | [34,42,80,96,111,122] |
| Diminished weight gain of the offspring from PND0 to 21. | [122] |
| Lower concentration of Zn, Fe and Cu in fetal liver, and Ca levels in fetal kidney. | [111,123] |
| Lower activities of hepatic estradiol metabolizing enzymes (17-β-hydroxysteroid and UDP glucoronyl transferase) in fetuses (GD20) and pups (PND21). | [111] |
| Decreased DNA and glycogen hepatic content at PND21. | [111] |
| At PND21, lower activities of alkaline phosphatase, acid phosphatase and Na+/K+ ATPase in kidney tissue. | [122] |
| Reduced anogenital index in pups at PND1 and 21. | [114] |
| Delayed hair appearance, testicular descent, palmar grasp and negative geotaxis in pups. | [114] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobo-Estrada, T.; Santoyo-Sánchez, M.; Thévenod, F.; Barbier, O. Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. Int. J. Mol. Sci. 2017, 18, 1590. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071590
Jacobo-Estrada T, Santoyo-Sánchez M, Thévenod F, Barbier O. Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. International Journal of Molecular Sciences. 2017; 18(7):1590. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071590
Chicago/Turabian StyleJacobo-Estrada, Tania, Mitzi Santoyo-Sánchez, Frank Thévenod, and Olivier Barbier. 2017. "Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models" International Journal of Molecular Sciences 18, no. 7: 1590. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071590