Comparison of Two Stationary Phases for the Determination of Phytosterols and Tocopherols in Mango and Its By-Products by GC-QTOF-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of GC-QTOF-MS Methods
2.2. Methods Validation
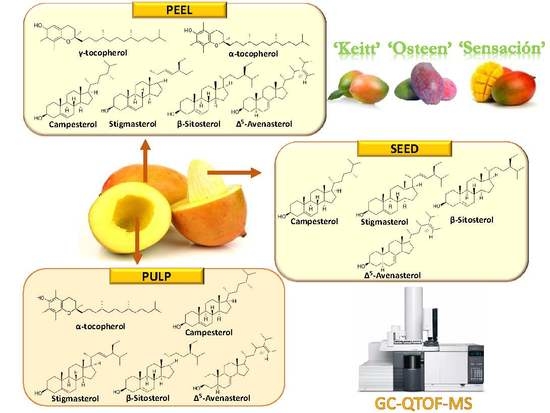
2.3. Identification of Phytosterols and Tocopherols of Mango Fractions
2.4. Quantification of Phytosterols and Tocopherols of Mango Fractions
3. Materials and Methods
3.1. Samples
3.2. Chemicals and Reagents
3.3. Isolation of Unsaponifiable Fraction by Hot Saponification
3.4. Determination of Phytosterols and Tocopherols by GC-QTOF-MS
3.4.1. HP-5ms Column
3.4.2. RTX-200MS Column
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ueda, M.; Sasaki, K.; Utsunomiya, N.; Inaba, K.; Shimabayashi, Y. Changes in physical and chemical properties during maturation of mango fruit (Mangifera indica L. “Irwin”) cultured in a plastic greenhouse. Food Sci. Technol. Res. 2000, 6, 299–305. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Soc. Technol. 2002, 12, 401–413. [Google Scholar] [CrossRef]
- Muchiri, D.R.; Mahungu, S.M.; Gituanja, S.N. Studies on mango (Mangifera indica L.) kernel fat of some Kenyan varieties in Meru. J. Am. Oil Chem. Soc. 2012, 89, 1567–1575. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Abdalla, A.E.M.; Darwish, S.M.; Ayad, E.H.E.; El-Hamahmy, R.M. Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem. 2007, 103, 1134–1140. [Google Scholar] [CrossRef]
- Vilela, C.; Santos, S.A.; Oliveira, L.; Camacho, J.F.; Cordeiro, N.; Freire, C.S.R.; Silvestre, A.J.D. The ripe pulp of Mangifera indica L.: A rich source of phytosterols and other lipophilic phytochemicals. Food Res. Int. 2013, 54, 1535–1540. [Google Scholar] [CrossRef]
- Peng, L.; Kawagoe, Y.; Hogan, P.; Delmer, D. Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 2002, 295, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Read, S.M.; Bacic, T. Plant biology: Prime time for cellulose. Science 2002, 295, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Lorencio, F.G. Status Nutricional de Carotenoides, Retinol y Tocoferoles en la Diabetes Mellitus Insulino Dependiente; Universidad Complutense de Madrid: Madrid, Spain, 2006. [Google Scholar]
- Ling, W.H.; Jones, P.J.H. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Mosaddik, A.; Gyawali, R.; Ahn, K.S.; Cho, S.K. Induction of apoptosis by ethanolic extract of mango peel and comparative analysis of the chemical constitutes of mango peel and flesh. Food Chem. 2012, 133, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Arogba, S.S. Physical, chemical and functional properties of Nigerian mango (Mangifera indica) kernel and its processed flour. J. Sci. Food Agric. 1997, 73, 321–328. [Google Scholar] [CrossRef]
- Solís-Fuentes, J.A.; Durán-de-Bazúa, M.C. Mango (Mangifera indica L.) Seed and Its Fats. In Nuts and seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 741–748. [Google Scholar]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.M.; Mohd Omar, A.K. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Abedin, M.Z.; Ghafoor, K.; Mohd Omar, A.K. Characterization of crystallization and melting profiles of blends of mango seed fat and palm oil mid-fraction as cocoa butter replacers using differential scanning calorimetry and pulse nuclear magnetic resonance. Food Res. Int. 2014, 55, 103–109. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Bouchet, P. Sterols, methyl sterols, triterpene alcohols and fatty acids of the kernel fat of different malagasy mango (Mangifera indica) varieties. J. Am. Oil Chem. Soc. 1984, 61, 1589–1593. [Google Scholar] [CrossRef]
- Dhara, R.; Bhattacharyya, D.K.; Ghosh, M. Analysis of sterol and other components present in unsaponifiable matters of mahua, sal and mango kernel oil. J. Oleo Sci. 2010, 59, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Jaganmohan Rao, L.; Prasada Rao, U.J.S. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Liu, F.; Guo, X.; Fu, X.; Li, T.; Liu, R.H. Phytochemical composition, cellular antioxidant capacity and antiproliferative activity in mango (Mangifera indica L.) pulp and peel. Int. J. Food Sci. Technol. 2017, 52, 817–826. [Google Scholar] [CrossRef]
- Rao, M.K.G.; Perkins, E.G. Identification and estimation of tocopherols and tocotrienols in vegetable oils using gas chromatography-mass spectrometry. J. Agric. Food Chem. 1972, 20, 240–245. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, W.; Wei, X.; Zhang, F.; Shen, C.; Wu, B.; Zhao, Z.; Liu, H.; Deng, X. Simultaneous determination of tocopherols and tocotrienols in vegetable oils by GC-MS. Anal. Methods 2016, 8, 7341–7346. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Pugajeva, I.; Czubinski, J.; Waśkiewicz, A.; Polewski, K. New insights regarding tocopherols in Arabica and Robusta species coffee beans: RP-UPLC-ESI/MSn and NP-HPLC/FLD study. J. Food Compos. Anal. 2014, 36, 117–123. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Soliven, A. Lipophilic bioactive compounds in the oils recovered from cereal by-products. J. Sci. Food Agric. 2016, 96, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Radenkovs, V.; Pugajeva, I.; Soliven, A.; Needs, P.W.; Kroon, P.A. Varied composition of tocochromanols in different types of bran: Rye, wheat, oat, spelt, buckwheat, corn, and rice. Int. J. Food Prop. 2016, 19, 1757–1764. [Google Scholar] [CrossRef]
- Pelillo, M.; Iafelice, G.; Marconi, E.; Caboni, M.F. Identification of plant sterols in hexaploid and tetraploid wheats using gas chromatography with mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Knights, B.A. Identification of plant sterols using combined GLC/mass spectrometry. J. Chromatogr. Sci. 1967, 5, 273–282. [Google Scholar] [CrossRef]
- Jin, J.; Warda, P.; Mu, H.; Zhang, Y.; Jie, L.; Mao, J.; Xie, D.; Huang, J.; Jin, Q.; Wang, X. Characteristics of mango kernel fats extracted from 11 China-specific varieties and their typically fractionated fractions. J. Am. Oil Chem. Soc. 2016, 93, 1115–1125. [Google Scholar] [CrossRef]
- Górnaś, P.; Seglina, D.; Lacis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Lace, B.; Lacis, G.; Segliņa, D. Tocochromanols composition in seeds recovered from different pear cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. LWT-Food Sci. Technol. 2015, 62, 104–107. [Google Scholar] [CrossRef]
- Górnaś, P. Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: Rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem. 2015, 172, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Esche, R.; Scholz, B.; Engel, K. Online LC−GC analysis of free sterols/stanols and intact steryl/stanyl esters in cereals. J. Agric. Food Chem. 2013, 61, 10932–10939. [Google Scholar] [CrossRef] [PubMed]
- Sweeley, C.C.; Bentley, R.; Makita, M.; Wells, W. Gas-liquid chromatography of trimethylsilyl derivatives of sugars and related substances. J. Am. Chem. Soc. 1963, 85, 2497–2507. [Google Scholar] [CrossRef]

| Analyte | Column | Calibration Range (μg/mL) | Calibration Equations | R2 | LOD (μg/mL) | LOQ (μg/mL) | Accuracy (% RSD) | ||
|---|---|---|---|---|---|---|---|---|---|
| LOD μg/mL | 50 μg/mL | 100 μg/mL | |||||||
| Campesterol | HP5 | LOD-100 | y = 175,991x – 692,519 | 0.9962 | 0.883 | 2.942 | 99.3 | 97.5 | 99.7 |
| RTX | LOD-100 | y = 245,482x – 89,617 | 0.9913 | 0.578 | 1.925 | 99.9 | 98.8 | 99.1 | |
| Stigmasterol | HP5 | LOD-100 | y = 172,642x – 699,942 | 0.9961 | 0.900 | 2.999 | 100.1 | 99.4 | 99.6 |
| RTX | LOD-100 | y = 325,223x – 200,715 | 0.9900 | 0.589 | 1.963 | 99.7 | 100.2 | 99.9 | |
| β-sitosterol | HP5 | LOD-100 | y = 119,251x + 444,578 | 0.9944 | 1.304 | 4.342 | 98.2 | 96.1 | 100.4 |
| RTX | LOD-100 | y = 180,878x – 101,182 | 0.9952 | 0.852 | 2.841 | 99.0 | 98.3 | 99.5 | |
| α-tocopherol | HP5 | LOD-100 | y = 124,615x – 807,670 | 0.9958 | 1.247 | 4.155 | 98.7 | 99.2 | 97.6 |
| RTX | LOD-100 | y = 186,088x – 178,465 | 0.9989 | 0.816 | 2.719 | 97.6 | 99.3 | 98.7 | |
| Compound | HP5 | RTX | ||||||
|---|---|---|---|---|---|---|---|---|
| Intraday RT | Intraday Peak Area | Interday RT | Interday Peak Area | Intraday RT | Intraday Peak Area | Interday RT | Interday Peak Area | |
| α-tocopherol | 0.06–0.10 | 0.84–1.97 | 0.17–0.23 | 2.53–3.14 | 0.01–0.03 | 0.99–2.41 | 0.015–0.023 | 1.27–2.14 |
| Campesterol | 0.04–0.09 | 0.05–0.08 | 0.019–0.22 | 1.35–1.76 | 0.005–0.02 | 0.98–1.49 | 0.009–0.017 | 1.51–1.99 |
| Stigmasterol | 0.02–0.13 | 0.47–0.68 | 0.20–022 | 1.29–1.54 | 0.02–0.03 | 0.85–1.73 | 0.013–0.027 | 1.67–1.96 |
| β-sitosterol | 0.02–0.07 | 0.52–0.71 | 0.19–0.23 | 1.31–1.50 | 0.01–0.03 | 0.97–1.4 | 0.014–0.025 | 1.87–2.61 |
| Peak | Proposed Compound | Retention Time (min) | m/z Experimental (M+) | m/z Calculated (M+) | Fragments | Molecular Formula | Pulp | Peel | Seed | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP5 | RTX | HP5 | RTX | ||||||||
| 1 | γ-tocopherol | 8.47 | 9.368 | 488.4010 | 488.4037 | 488.4050 | 263.1471/223.1152 | C31H56O2Si | - | √ | - |
| 2 | α-tocopherol | 11.282 | 10.518 | 502.4170 | 502.4170 | 502.4206 | 277.1631/237.1314 | C32H58O2Si | √ | √ | √ |
| 3 | Campesterol | 14.201 | 11.860 | 472.4074 | 472.4095 | 472.4100 | 457.3884/382.3610/367.3373/343.3367/253.0179/213.1652/129.0732 | C31H56OSi | √ | √ | √ |
| 4 | Stigmasterol | 15.110 | 12.040 | 484.4070 | 484.4073 | 484.4100 | 469.3874/394.3609/379.3337/355.3370/343.3362/255.2116/253.0182/213.1650/129.0731 | C32H56OSi | √ | √ | √ |
| 5 | β-sitosterol | 17.029 | 12.882 | 486.4220 | 486.4243 | 486.4257 | 471.4044/396.3770/381.3535/343.3363/357.3531/255.2121/253.0179/213.1653/129.0745 | C32H58OSi | √ | √ | √ |
| 6 | Δ5-avenasterol | 17.523 | 12.999 | 484.4070 | 484.4075 | 484.4100 | 469.3871/394.3605/386.2977/379.3334/355.3368/343.3365/255.2107/253.0182/213.0180/129.0737 | C32H56OSi | √ | √ | √ |
| Compounds | Peel | Pulp | Seed | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Keitt | Osteen | Sensación | Keitt | Osteen | Sensación | Keitt | Osteen | Sensación | ||
| γ-tocopherol | HP5 | 6.13 ± 0.41b | 7.09 ± 0.17c | 14.41 ± 0.48d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| RTX | 5.19 ± 0.20b | 6.22 ± 0.07c | 17.73 ± 0.60d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| α-tocopherol | HP5 | 20.99 ± 0.74f | 18.47 ± 0.39e | 43.45 ± 1.38g | 6.72 ± 0.17c | 5.16 ± 0.14b | 14.81 ± 0.26d | 0.98 ± 0.04a | 0.87 ± 0.09a | 1.02 ± 0.08a |
| RTX | 22.39 ± 1.06e | 16.61 ± 0.31b | 49.86 ± 1.20f | 6.62 ± 0.69d | 4.71 ± 0.17c | 16.56 ± 0.40b | 0.78 ± 0.08a | 0.75 ± 0.08a | 0.93 ± 0.09a | |
| TOTAL TOCOPHEROLS | HP5 | 27.12 ± 1.15c | 25.56 ± 0.56c | 57.86 ± 1.86e | 6.72 ± 0.17b | 5.16 ± 0.14b | 14.81 ± 0.26d | 0.98 ± 0.04a | 0.87 ± 0.09a | 1.02 ± 0.08a |
| RTX | 27.58 ± 1.26f | 22.83 ± 0.38e | 67.59 ± 1.80g | 6.62 ± 0.69c | 4.71 ± 0.17b | 16.56 ± 0.40d | 0.78 ± 0.08a | 0.75 ± 0.08a | 0.93 ± 0.09a | |
| Campesterol | HP5 | 62.89 ± 0.76c | 58.80 ± 1.43b | 57.16 ± 1.57b | 51.84 ± 0.43f | 45.06 ± 1.11e | 62.62 ± 0.73c | 39.09 ± 1.36d | 25.53 ± 0.32a | 27.79 ± 1.56a |
| RTX | 58.25 ± 2.38c | 54.01 ± 2.51ac | 53.06 ± 2.30a | 52.82 ± 1.71a | 45.10 ± 0.78e | 63.04 ± 1.78f | 39.23 ± 1.44d | 25.50 ± 0.89b | 28.97 ± 2.18b | |
| Stigmasterol | HP5 | 47.84 ± 4.54c | 42.40 ± 0.75b | 61.62 ± 0.94f | 8.48 ± 0.42a | 7.21 ± 0.40a | 14.23 ± 0.41f | 47.73 ± 1.87c | 42.02 ± 0.55b | 35.37 ± 1.31e |
| RTX | 57.38 ± 1.96b | 42.54 ± 1.96f | 86.09 ± 3.31g | 14.61 ± 1.09d | 8.15 ± 0.32c | 20.77 ± 1.51e | 57.33 ± 2.14b | 51.58 ± 1.04a | 49.82 ± 3.67a | |
| β-sitosterol | HP5 | 442.92 ± 18.11e | 473.89 ± 9.07d | 472.89 ± 9.07d | 180.49 ± 3.59a | 172.24 ± 1.15a | 239.88 ± 1.83b | 299.56 ± 8.47c | 244.61 ± 3.21b | 293.19 ± 5.83c |
| RTX | 504.65 ± 12.77c | 565.28 ± 15.34f | 497.59 ± 11.01c | 217.66 ± 3.02a | 218.29 ± 2.58a | 290.25 ± 11.43b | 382.73 ± 6.23d | 307.68 ± 6.00b | 421.81 ± 11.03e | |
| Δ5-avenasterol | HP5 | 12.23 ± 0.91a | 18.91 ± 0.44b | 11.55 ± 0.93a | 13.15 ± 0.36a | 20.52 ± 1.44b | 26.79 ± 0.82c | 27.31 ± 0.68c | 5.36 ± 0.09d | 34.30 ± 2.39e |
| RTX | 22.98 ± 1.76ad | 30.73 ± 2.38ab | 16.16 ± 0.96c | 18.13 ± 1.28cd | 25.94 ± 2.82ab | 27.76 ± 2.29ab | 30.08 ± 2.57b | 7.87 ± 0.78e | 43.27 ± 4.55f | |
| Total Phytosterols | HP5 | 565.88 ± 24.32e | 594.00 ± 11.69f | 603.41 ± 12.37g | 253.96 ± 4.80a | 245.03 ± 4.10a | 343.52 ± 3.79d | 413.69 ± 12.38b | 317.52 ± 4.17c | 390.65 ± 11.09b |
| RTX | 643.26 ± 18.87f | 692.56 ± 22.19c | 652.90 ± 21.98c | 311.44 ± 7.10a | 297.48 ± 6.50a | 401.82 ± 17.01b | 509.37 ± 12.38d | 392.63 ± 8.71b | 543.87 ± 21.43e | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cobo, A.; Martín-García, B.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Comparison of Two Stationary Phases for the Determination of Phytosterols and Tocopherols in Mango and Its By-Products by GC-QTOF-MS. Int. J. Mol. Sci. 2017, 18, 1594. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071594
López-Cobo A, Martín-García B, Segura-Carretero A, Fernández-Gutiérrez A, Gómez-Caravaca AM. Comparison of Two Stationary Phases for the Determination of Phytosterols and Tocopherols in Mango and Its By-Products by GC-QTOF-MS. International Journal of Molecular Sciences. 2017; 18(7):1594. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071594
Chicago/Turabian StyleLópez-Cobo, Ana, Beatriz Martín-García, Antonio Segura-Carretero, Alberto Fernández-Gutiérrez, and Ana María Gómez-Caravaca. 2017. "Comparison of Two Stationary Phases for the Determination of Phytosterols and Tocopherols in Mango and Its By-Products by GC-QTOF-MS" International Journal of Molecular Sciences 18, no. 7: 1594. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071594