Comparative In Vitro Controlled Release Studies on the Chronobiotic Hormone Melatonin from Cyclodextrins-Containing Matrices and Cyclodextrin: Melatonin Complexes
Abstract
:1. Introduction
2. Results and Discussion
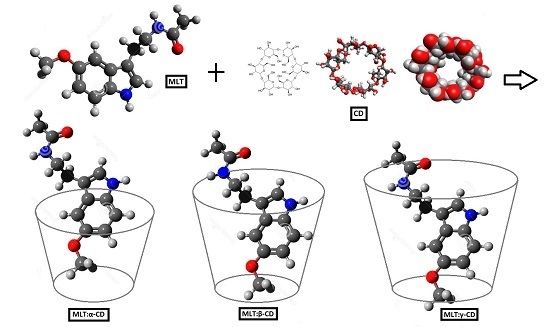
2.1. Estimation of MLT-CD Complexes’ Stoichiometry
2.2. Determination of MLT-CD Complexes Stability Constants
2.3. Monitoring of MLT Complexes with UV-Vis
2.4. Confirmation of MLT Complex Formation via NMR Spectroscopy
2.5. Tablet Formulation and Characterization
2.6. Dissolution Studies
3. Materials and Methods
3.1. Determination of MLT–CD Complex Stoichiometry
3.2. Determination of Stability Constants
3.3. Preparation of MLT:CD Complexes
3.4. Instrumentation for Monitoring Host–Guest Interactions
3.5. Materials Used for the Preparation of Matrix Tablets
3.6. Preparation of Matrix Tablets
3.7. Characterization of Tablets: Tablet Thickness, Hardness, and Friability
3.8. In Vitro Dissolution Studies
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Brzezinski, A. Melatonin in humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [PubMed]
- Turek, F.W.; Gillette, M.U. Melatonin, sleep and circadian rhythms: Rationale for development of specific melatonin agonists. Sleep Med. 2004, 5, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Agarwal, S.P.; Khanna, R. Modified release bi-layer tablet of melatonin using β-cyclodextrin. Pharmazie 2003, 58, 642–644. [Google Scholar] [PubMed]
- Lane, E.A.; Moss, H.B. Pharmacokinetics of melatonin in man: First pass hepatic metabolism. J. Clin. Endocrinol. Metab. 1985, 61, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Ioannidou, V.; Vertzoni, M.; Tsotinis, A.; Afroudakis, P.; Sugden, D. Controlled release from solid pharmaceutical formulations of two nalkanoyl-4-methoxybicyclo [4.2.0] octa-1,3,5-trien-7-ethanamines with melatoninergic activity. Lett. Drug Des. Discov. 2015, 12, 259–262. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A.; Pareli, I.; Zampakola, A.; Konstantinidou, S. An account of modified release of melatonin from compression-coated, uncoated and bilayer tablets. J. Pharm. Pharm. Sci. 2016, 1, 10–14. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A.; Konstantinidou, S.; Dotsikas, Y. Optimization of controlled release matrix formulation of melatonin via experimental design. J. Pharm. Drug Deliv. Res. 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Zampakola, A.; Siamidi, A.; Pippa, N.; Demetzos, C.; Vlachou, M. Chronobiotic hormone melatonin: Comparative in vitro release studies from matrix tablets and liposomal formulations. Lett. Drug Des. Discov. 2017, 14, 476–480. [Google Scholar] [CrossRef]
- Lee, B.J.; Ryu, S.G.; Cui, J.H. Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug Dev. Ind. Pharm. 1999, 25, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Kumar, C.K.A.; Sravanthi, V.V.N. Cyclodextrins as drug carrier molecule: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Babu, R.J.; Dayal, P.; Singh, M. Effect of Cyclodextrins on the complexation and nasal permeation of melatonin. Drug Deliv. 2008, 15, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Ogawa, Y.; Nakayama, H. Inclusion complexes of melatonin with modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 217–224. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, Z.H.; Sun, E.; Jia, X.B. Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, J.; Wu, J. Study on the inclusion interactions of β-cyclodextrin and its derivative with dyes by spectrofluorimetry and its analytical application. Talanta 2007, 72, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, A.; Másson, M.; Loftsson, T. Self association and cyclodextrin solubilization of NSAIDs. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 213–218. [Google Scholar]
- Fragouli, I. Controlled Release of Melatonin from Solid Pharmaceutical Formulations in the Presence of Cyclodextrins as Excipients and as Complexes. Mater’s Thesis, National and Kapodistrian University of Athens, Athens, Greece, June 2008, unpublished work. [Google Scholar]
- Bongiorno, D.; Ceraulo, L.; Mele, A.; Panzeri, W.; Selva, A.; Liveri, V. Structural and physicochemical characterization of the inclusion complexes of cyclomaltooligosaccharides (cyclodextrins) with melatonin. Carbohydr. Res. 2002, 337, 743–754. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.N.; Kuchekar, B.S. Preparation, physicochemical characterization, dissolution and formulation studies of telmisartan cyclodextrin inclusion complexes. Asian J. Pharm. 2010, 1, 52–59. [Google Scholar] [CrossRef]
- Dotsikas, Y.; Kontopanou, E.; Allagiannis, C.; Loukas, Y.L. Interaction of 6-P-toluidinylnaphthalene-2-sulphonate with β-cyclodextrin. J. Pharm. Biomed. Anal. 2000, 23, 997–1003. [Google Scholar] [CrossRef]
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin inclusion complex to improve physicochemical properties of pipemidic acid: Characterization and bioactivity evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar] [CrossRef] [PubMed]
- Dubes, A.; Degobert, C.; Fessi, H.; Parrot-Lopez, H. Synthesis and characterization of sulfated amphiphilic α-, β- and γ-cyclodextrins: Application to the complexation of acyclovir. Carbohydr. Res. 2003, 338, 2185–2193. [Google Scholar] [CrossRef]
- Reddy, N.M.; Rehana, T.; Ramakrishna, S.; Chowdary, K.P.R.; Diwan, P. β-Cyclodextrin complexes of celecoxib: Molecular-modeling, characterization and dissolution studies. AAPS PharmSci 2004, 6, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Rinaki, E.; Dokoumetzidis, A.; Macheras, P. The mean dissolution time depends on the dose/solubility ratio. Pharm. Res. 2003, 20, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Doelker, G.F.P.; Peppas, N.A. Mechanism of potassium chloride from compressed, hydrophilic, polymeric matrices: Effect of entrapped air. J. Pharm. Sci. 1983, 72, 1189–1191. [Google Scholar] [CrossRef] [PubMed]












| Complex | Slope | Intercept | r2 | K (M−1) |
|---|---|---|---|---|
| MLT and α-CD | 23.30 × 10−3 | 23.623 | 0.993 | 1006.90 |
| MLT and β-CD | 15.00 × 10−3 | 27.716 | 0.995 | 1847.70 |
| MLT and S-β-CD | 8.00 × 10−3 | 37.160 | 0.990 | 4645.00 |
| MLT and γ-CD | 22.70 × 10−3 | 37.185 | 0.997 | 1638.00 |
| MLT and HP-α-CD | 20.70 × 10−3 | 59.149 | 0.991 | 2857.44 |
| MLT and HP-β-CD | 57.98 × 10−5 | 13.969 | 0.995 | 24,092.80 |
| MLT and HP-γ-CD | 9.70 × 10−3 | 52.689 | 0.991 | 5431.86 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLT | 2 | 2 | 2 | 2 | 2 | 2 | 2 | - | - | - | - | - | - | - |
| α-CD | 8.8 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| β-CD | - | 10.2 | - | - | - | - | - | - | - | - | - | - | - | - |
| S-β-CD | - | - | 22.5 | - | - | - | - | - | - | - | - | - | - | - |
| γ-CD | - | - | - | 11.7 | - | - | - | - | - | - | - | - | - | - |
| HP-α-CD | - | - | - | - | 10.6 | - | - | - | - | - | - | - | - | - |
| HP-β-CD | - | - | - | - | - | 13.9 | - | - | - | - | - | - | - | - |
| HP-γ-CD | - | - | - | - | - | - | 14.3 | - | - | - | - | - | - | - |
| MLT:α-CD | - | - | - | - | - | - | - | 10.8 | - | - | - | - | - | - |
| MLT:β-CD | - | - | - | - | - | - | - | - | 12.2 | - | - | - | - | - |
| MLT:S-β-CD | - | - | - | - | - | - | - | - | - | 24.5 | - | - | - | - |
| MLT:γ-CD | - | - | - | - | - | - | - | - | - | - | 13.7 | - | - | - |
| MLT:HP-α-CD | - | - | - | - | - | - | - | - | - | - | - | 12.6 | - | - |
| MLT:HP-β-CD | - | - | - | - | - | - | - | - | - | - | - | - | 15.9 | - |
| MLT:HP-γ-CD | - | - | - | - | - | - | - | - | - | - | - | - | - | 16.3 |
| HPMC K 15M | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Avicel PH 102 | 127.2 | 125.8 | 113.5 | 124.3 | 125.4 | 122.1 | 121.7 | 127.2 | 125.8 | 113.5 | 124.3 | 125.4 | 122.1 | 121.7 |
| Sodium Alginate | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Mg.Stearate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| pH 1.2 | pH 7.4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | t20% | t50% | t90% | MDT | D.E. (%) | n | t20% | t50% | t90% | MDT | D.E. (%) | |
| F1 | 1.03 | 370 | b | b | 208.93 | 15.76 | 0.49 | 95 | b | b | 121.83 | 38.41 |
| F2 | 0.49 | 29 | 315 | b | 149.42 | 66.00 | 0.62 | 95 | 165 | b | 163.96 | 61.70 |
| F3 | 0.45 | 16 | 90 | 240 | 103.25 | 69.70 | 0.84 | 23 | 99 | 240 | 109.50 | 62.30 |
| F4 | 0.85 | 95 | 250 | b | 167.73 | 44.13 | 0.77 | 82 | 240 | b | 157.28 | 48.25 |
| F5 | 0.47 | 22 | 130 | b | 121.10 | 68.67 | 0.70 | 24 | 131 | 312 | 139.56 | 75.43 |
| F6 | 0.45 | 15 | 78 | 400 | 92.16 | 78.60 | 0.45 | 10 | 26 | 110 | 77.58 | 63.00 |
| F7 | 0.64 | 25 | 168 | b | 124.23 | 56.50 | 1.05 | 49 | 126 | 302 | 143.13 | 78.26 |
| F8 | 0.62 | 45 | 210 | b | 157.71 | 55.79 | 0.78 | 60 | 180 | 480 | 162.73 | 60.84 |
| F9 | a | 9 | 22 | 120 | 70.89 | 70.90 | a | 15 | 75 | 400 | 98.60 | 46.50 |
| F10 | 0.52 | 9 | 23 | 150 | 55.90 | 59.38 | 0.77 | 23 | 92 | 170 | 95.99 | 69.40 |
| F11 | a | 25 | 183 | b | 143.12 | 79.60 | 0.82 | 59 | 155 | 335 | 154.41 | 58.60 |
| F12 | 0.68 | 25 | 105 | 450 | 114.88 | 73.24 | 0.95 | 32 | 98 | 115 | 108.34 | 85.57 |
| F13 | a | 11 | 27 | 80 | 65.72 | 76.80 | a | 8 | 22 | 85 | 45.97 | 54.90 |
| F14 | 0.62 | 25 | 138 | 180 | 113.13 | 68.81 | 0.81 | 28 | 116 | 230 | 119.22 | 91.97 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlachou, M.; Papamichael, M.; Siamidi, A.; Fragouli, I.; Afroudakis, P.A.; Kompogennitaki, R.; Dotsikas, Y. Comparative In Vitro Controlled Release Studies on the Chronobiotic Hormone Melatonin from Cyclodextrins-Containing Matrices and Cyclodextrin: Melatonin Complexes. Int. J. Mol. Sci. 2017, 18, 1641. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081641
Vlachou M, Papamichael M, Siamidi A, Fragouli I, Afroudakis PA, Kompogennitaki R, Dotsikas Y. Comparative In Vitro Controlled Release Studies on the Chronobiotic Hormone Melatonin from Cyclodextrins-Containing Matrices and Cyclodextrin: Melatonin Complexes. International Journal of Molecular Sciences. 2017; 18(8):1641. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081641
Chicago/Turabian StyleVlachou, Marilena, Marianna Papamichael, Angeliki Siamidi, Irene Fragouli, Pandelis A. Afroudakis, Rodanthi Kompogennitaki, and Yannis Dotsikas. 2017. "Comparative In Vitro Controlled Release Studies on the Chronobiotic Hormone Melatonin from Cyclodextrins-Containing Matrices and Cyclodextrin: Melatonin Complexes" International Journal of Molecular Sciences 18, no. 8: 1641. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081641