Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes
Abstract
:1. Introduction
2. Chemical Synthesis of Nucleic Acid Aptamers
2.1. Synthesis of DNA Aptamers
2.2. Synthesis of RNA Aptamers
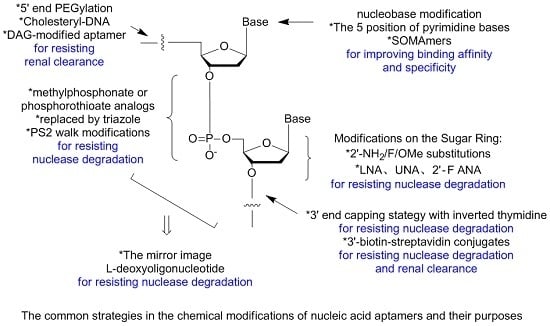
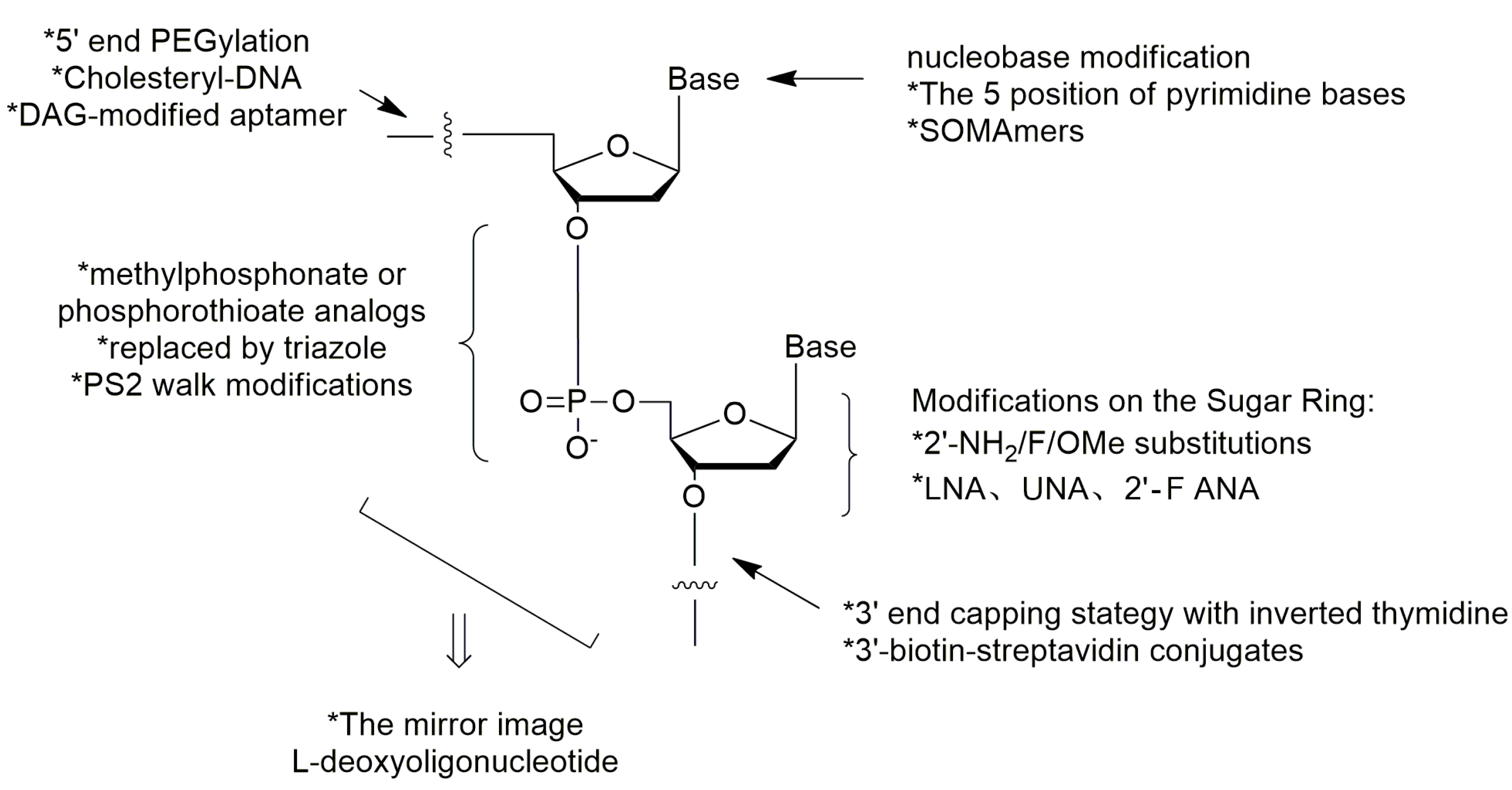
3. Modifications of Nucleic Acid Aptamers
3.1. Aptamer Derivatives for Resisting Nuclease Degradation
3.1.1. Terminal 3′–3′ and 5′–5′ Internucleotide Linkage
3.1.2. 3′-Biotin Conjugates
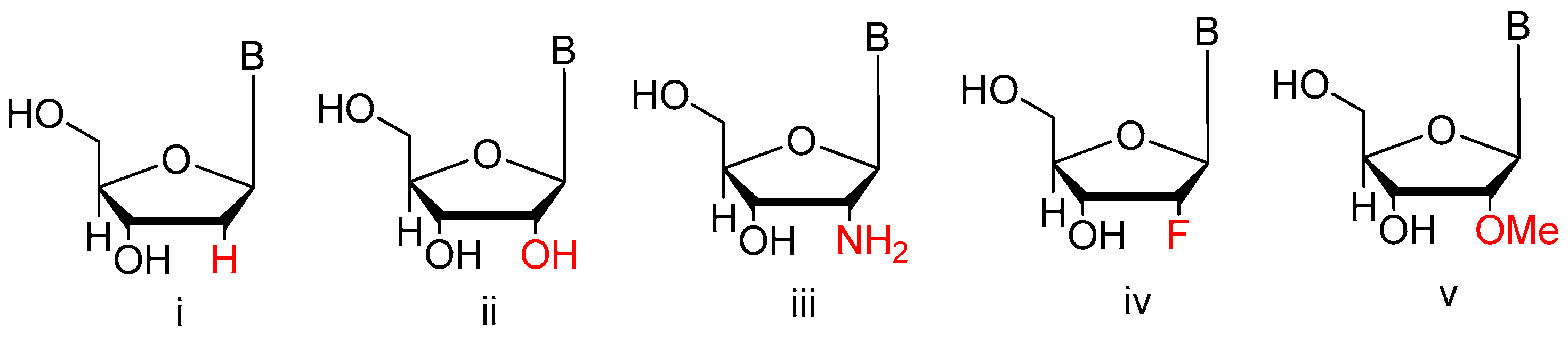
3.1.3. Modifications on the Sugar Ring
2′-Substitutions
LNA, UNA, 2′-F ANA
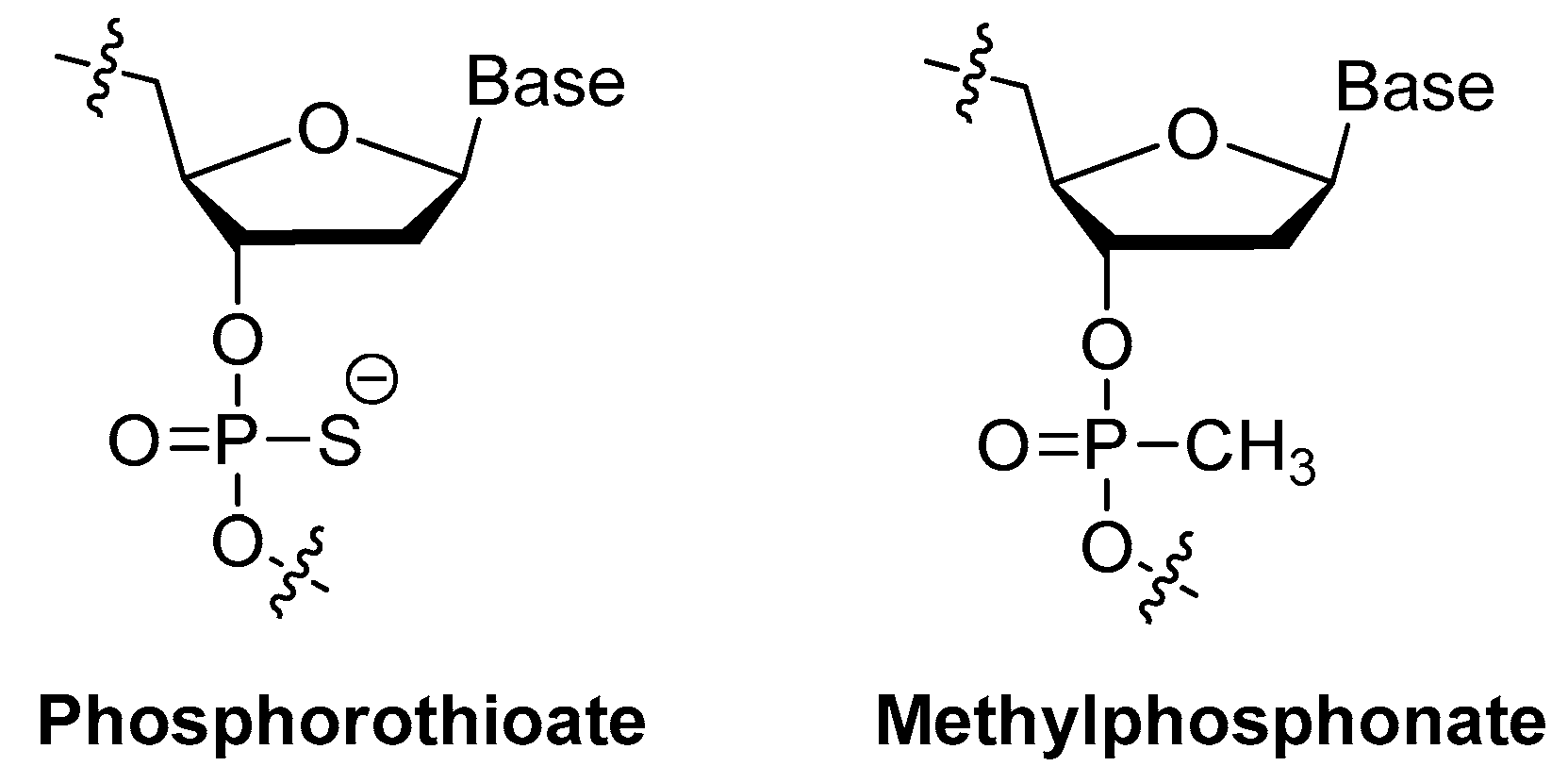
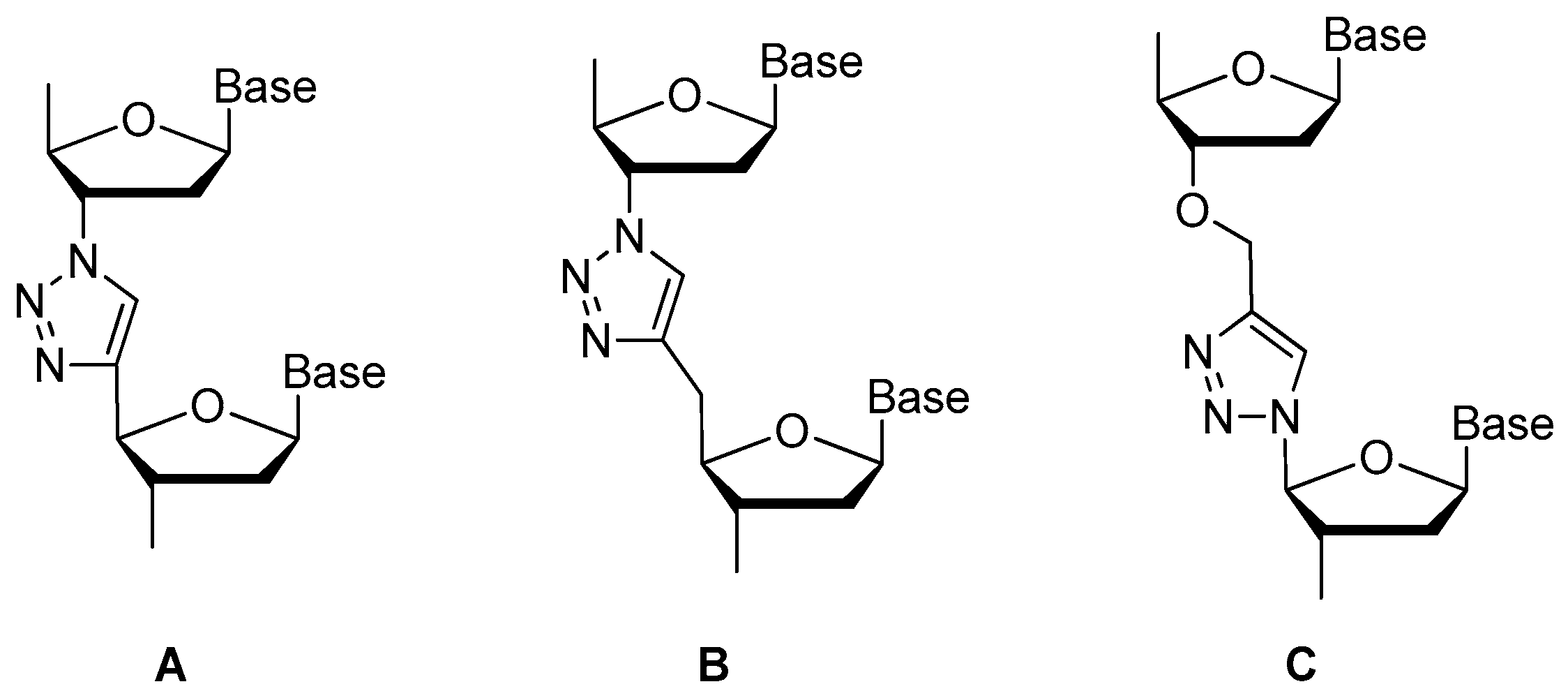
3.1.4. Modifications on the Phosphodiester Linkage
Methylphosphonate or Phosphorothioate
Replaced by Triazole
3.1.5. The Mirror Image l-DNA
3.2. Aptamer Derivatives for Resisting Renal Clearance
3.2.1. 5′-End with Cholesterol
3.2.2. 5′-End with Dialkyl Lipids
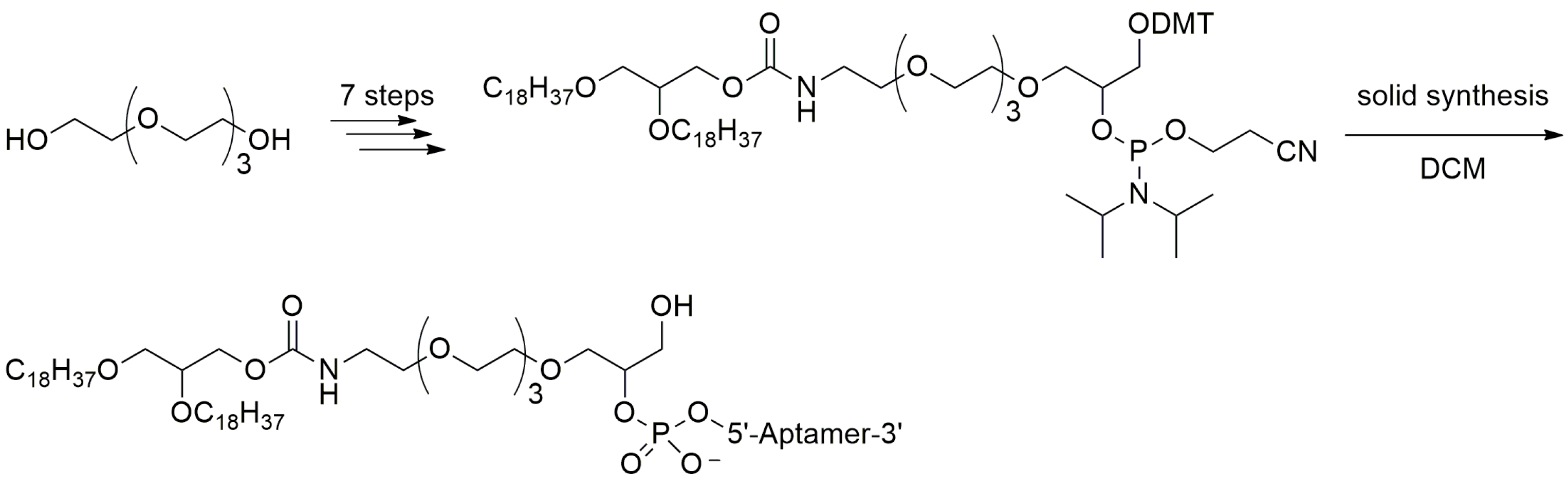
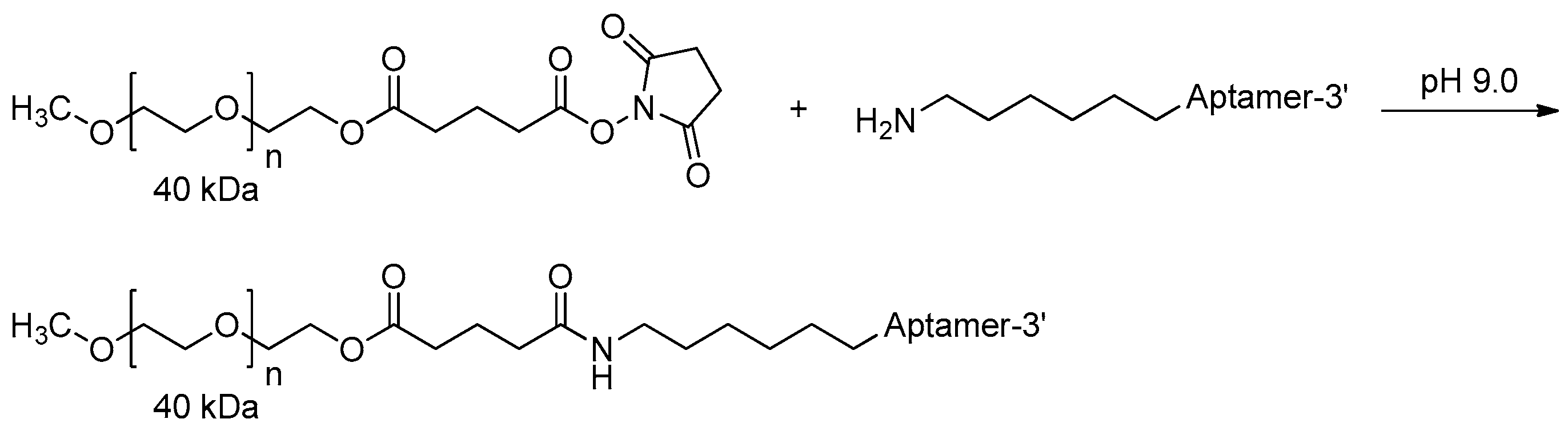
3.2.3. 5′-End PEGylation
3.3. Aptamer Derivatives for Improving Binding Affinity and Target Selectivity
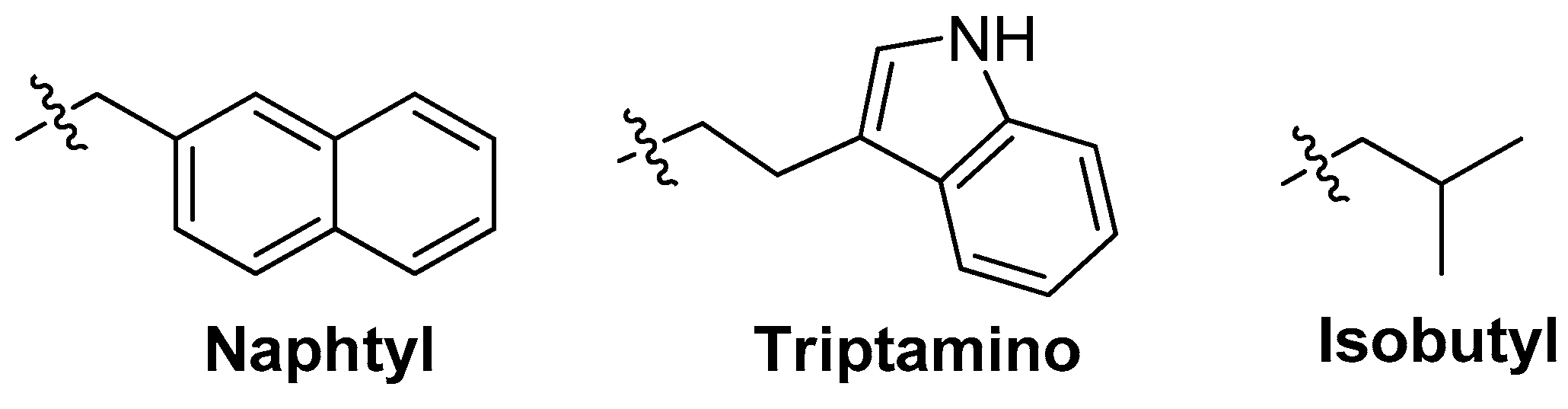
3.3.1. Modifications on the Bases; SOMAmers
3.3.2. Crystal Structure Based Modifications
3.3.3. NMR Spectroscopy Guided Aptamer Optimization
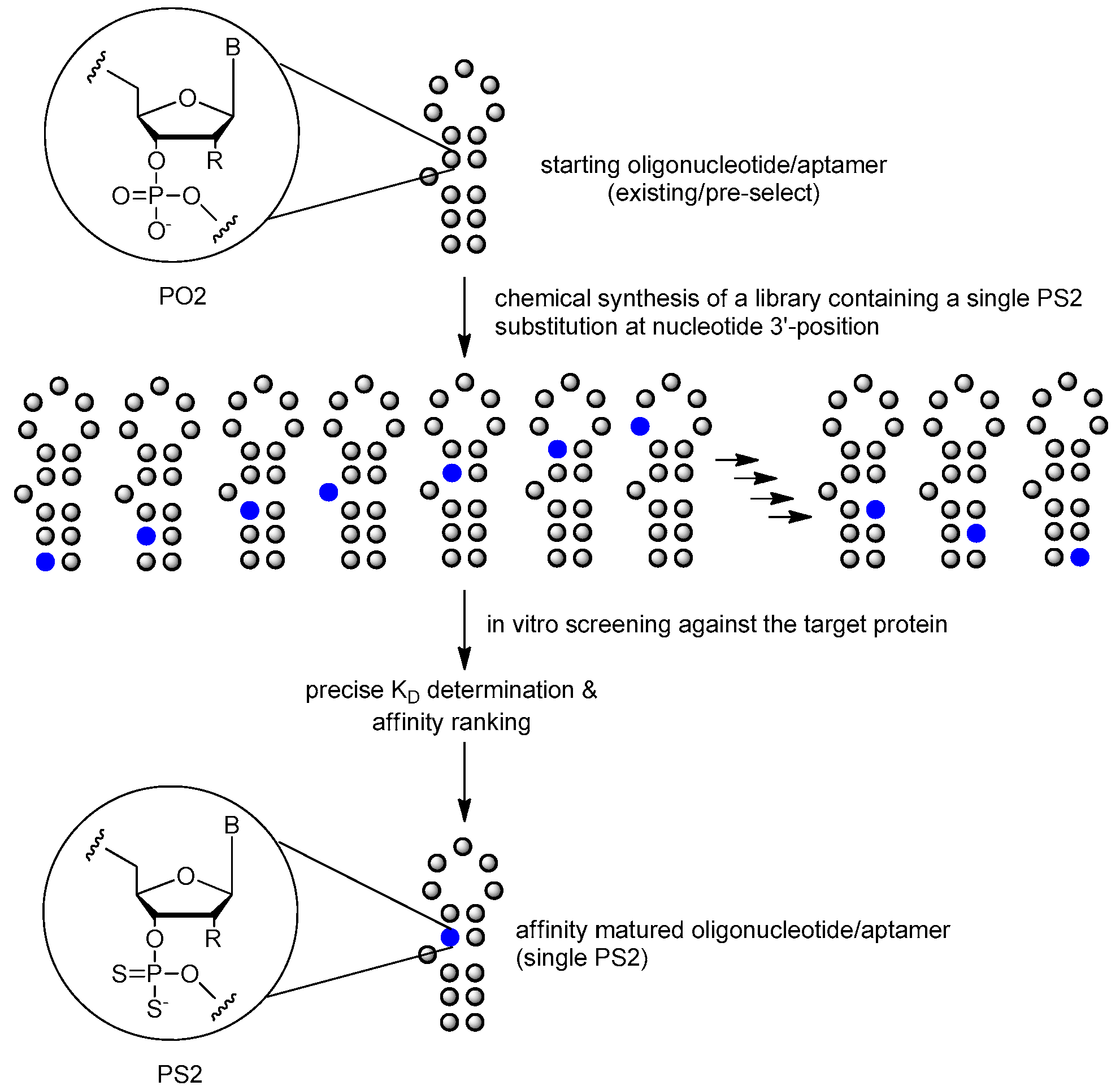
3.3.4. PS2 Walk
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, A.D.; Davies, D.R.; Janjic, N. Embracing proteins: Structural themes in aptamer-protein complexes. Curr. Opin. Struct. Biol. 2016, 36, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ashrafuzzaman, M. Aptamers as both drugs and drug-carriers. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Dass, C.R.; Saravolac, E.G.; Li, Y.; Sun, L.Q. Cellular uptake, distribution, and stability of 10–23 deoxyribozymes. Antisense Nucleic Acid Drug Dev. 2002, 12, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.V.; Blanchard, K.; Shaw, L.; Jensen, K.; Lockridge, J.A.; Dickinson, B.; McSwiggen, J.A.; Vargeese, C.; Bowman, K.; Shaffer, C.S.; et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology 2005, 41, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.C.; Tidmarsh, G.F.; Bock, L.C.; Toole, J.J.; Leung, L.L. In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood 1993, 81, 3271–3276. [Google Scholar] [PubMed]
- Pagratis, N.C.; Bell, C.; Chang, Y.F.; Jennings, S.; Fitzwater, T.; Jellinek, D.; Dang, C. Potent 2′-amino-, and 2′-fluoro-2′-deoxyribonucleotide RNA inhibitors of keratinocyte growth factor. Nat. Biotechnol. 1997, 15, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.D.; Egli, M.; Cox, N.; Mercier, K.; Conde, J.N.; Pallan, P.S.; Mizurini, D.M.; Sierant, M.; Hibti, F.E.; Hassell, T.; et al. Evoking picomolar binding in RNA by a single phosphorodithioate linkage. Nucleic Acids Res. 2016, 44, 8052–8064. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Ortigao, J.R.; Rosch, H.; Montenarh, M.; Frohlich, A.; Seliger, H. Oligonucleotide analogs with terminal 3′, 3′-and 5′, 5′-internucleotidic linkages as antisense inhibitors of viral replication. Antisense Res. Dev. 1991, 1, 380. [Google Scholar] [CrossRef]
- Ng, E.W.; Shima, D.T.; Calias, P.; Cunningham, E.T., Jr.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Doggrell, S.A. Pegaptanib: The first antiangiogenic agent approved for neovascular macular degeneration. Expert Opin. Pharmacother. 2005, 6, 1421–1423. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.L.; Martin, D.F.; Kirkpatrick, P. Pegaptanib sodium. Nat. Rev. Drug Discov. 2005, 4, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Kiire, C.A.; Morjaria, R.; Rudenko, A.; Fantato, A.; Smith, L.; Smith, A.; Chong, V. Intravitreal pegaptanib for the treatment of ischemic diabetic macular edema. Clin. Ophthalmol. 2015, 9, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A. Technology evaluation: Pegaptanib, Eyetech/Pfizer. Curr. Opin. Mol. Ther. 2003, 5, 673–679. [Google Scholar] [PubMed]
- Caruthers, M.H.; Barone, A.D.; Beaucage, S.L.; Dodds, D.R.; Fisher, E.F.; McBride, L.J.; Matteucci, M.; Stabinsky, Z.; Tang, J.Y. Chemical synthesis of deoxyoligonucleotides by the phosphoramidite method. Methods Enzymol. 1987, 154, 287–313. [Google Scholar] [PubMed]
- Sproat, B.S. RNA synthesis using 2′-O-(tert-butyldimethylsilyl) protection. Oligonucleotide Synth. 2005, 17–31. [Google Scholar]
- Shum, K.T.; Tanner, J.A. Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase. Chembiochem 2008, 9, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.P.; Kent, K.; Bird, J.; Fishback, J.; Froehler, B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991, 19, 747–750. [Google Scholar] [CrossRef] [PubMed]
- De Smidt, P.C.; le Doan, T.; de Falco, S.; van Berkel, T.J. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991, 19, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Kaluzhny, D.; Shchyolkina, A.; Borisova, O.; Smirnov, I.; Pozmogova, G. Conformation and thermostability of oligonucleotide d(GGTTGGTGTGGTTGG) containing thiophosphoryl internucleotide bonds at different positions. Biophys. Chem. 2010, 146, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pozmogova, G.; Zaitseva, M.; Smirnov, I.; Shvachko, A.; Murina, M.; Sergeenko, V. Anticoagulant effects of thioanalogs of thrombin-binding DNA-aptamer and their stability in the plasma. Bull. Exp. Biol. Med. 2010, 150, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.G.; Damha, M.J. G-quadruplex induced stabilization by 2′-deoxy-2′-fluoro-d-arabinonucleic acids (2′F-ANA). Nucleic Acids Res. 2007, 35, 4977–4988. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kang, H.; Ryu, S.H.; Lee, D.S.; Lee, J.H.; Kim, S. Bioimaging of nucleolin aptamer-containing 5-(N-benzylcarboxyamide)-2′-deoxyuridine more capable of specific binding to targets in cancer cells. J. Biomed. Biotechnol. 2010, 2010, 168306. [Google Scholar] [CrossRef] [PubMed]
- Pallan, P.S.; Yang, X.; Sierant, M.; Abeydeera, N.D.; Hassell, T.; Martinez, C.; Janicka, M.; Nawrot, B.; Egli, M. Crystal structure, stability and Ago2 affinity of phosphorodithioate-modified RNAs. Rsc. Adv. 2014, 4, 64901–64904. [Google Scholar] [CrossRef]
- Ashley, S.L.; Xia, M.; Murray, S.; O’Dwyer, D.N.; Grant, E.; White, E.S.; Flaherty, K.R.; Martinez, F.J.; Moore, B.B. Six-SOMAmer Index relating to immune, protease and angiogenic functions predicts progression in IPF. PLoS ONE 2016, 11, e0159878. [Google Scholar] [CrossRef] [PubMed]
- Eid, C.; Palko, J.W.; Katilius, E.; Santiago, J.G. Rapid slow off-rate modified aptamer (SOMAmer)-based detection of C-reactive protein using isotachophoresis and an ionic spacer. Anal. Chem. 2015, 87, 6736–6743. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Wang, X.; Diaz, A.; Goos-Root, D.M.; Bock, C.; Vaught, J.D.; Sun, W.; Strom, C.M. Measurement of cetuximab and panitumumab-unbound serum EGFR extracellular domain using an assay based on slow off-rate modified aptamer (SOMAmer) reagents. PLoS ONE 2013, 8, e71703. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.M.; Lewis, S.D.; Kurz, M.; Boomer, R.M.; Thompson, K.M.; Wilson, C.; McCauley, T.G. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 2004, 21, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.R.; Chang, Y.F.; O’Connell, D.; Weigand, L.; Ringquist, S.; Parma, D.H. Anti-l-selectin aptamers: Binding characteristics, pharmacokinetic parameters, and activity against an intravascular target in vivo. Antisense Nucleic Acid Drug Dev. 2000, 10, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.C.; DeFeo-Fraulini, T.; Hutabarat, R.M.; Horvath, C.J.; Merlino, P.G.; Marsh, H.N.; Healy, J.M.; Boufakhreddine, S.; Holohan, T.V.; Schaub, R.G. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 2007, 116, 2678–2686. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, L.; Swinkels, D.; John, A.; Schwoebel, F.; Fliegert, F.; Summo, L.; Vauleon, S.; Laarakkers, J.; Riecke, K.; Pickkers, P. Randomized double-blind placebo-controlled PK/PD study on the effects of a single intravenous dose of the anti-hepcidin Spiegelmer NOX-H94 on serum iron during experimental human endotoxemia. Crit. Care 2013, 17, P352. [Google Scholar]
- Dougan, H.; Lyster, D.M.; Vo, C.V.; Stafford, A.; Weitz, J.I.; Hobbs, J.B. Extending the lifetime of anticoagulant oligodeoxynucleotide aptamers in blood. Nucl. Med. Biol. 2000, 27, 289–297. [Google Scholar] [CrossRef]
- Dellinger, D.J.; Betley, J.R.; Wyrzykiewicz, T.K.; Caruthers, M.H. Synthesis of DNA using a new two-step cycle. Oligonucleotide Synth. 2005, 288, 1–16. [Google Scholar]
- Beaucage, S.L.; Caruthers, M.H. Synthetic strategies and parameters involved in the synthesis of oligodeoxyribonucleotides according to the phosphoramidite method. Curr. Protoc. Nucleic Acid Chem. 2001. [Google Scholar] [CrossRef]
- Maier, K.E.; Levy, M. From selection hits to clinical leads: Progress in aptamer discovery. Mol. Ther. Methods Clin. Dev. 2016, 5, 16014. [Google Scholar] [CrossRef] [PubMed]
- Usman, N.; Ogilvie, K.; Jiang, M.; Cedergren, R. The automated chemical synthesis of long oligoribuncleotides using 2′-O-silylated ribonucleoside 3′-O-phosphoramidites on a controlled-pore glass support: Synthesis of a 43-nucleotide sequence similar to the 3′-half molecule of an Escherichia coli formylmethionine tRNA. J. Am. Chem. Soc. 1987, 109, 7845–7854. [Google Scholar]
- Shiba, Y.; Masuda, H.; Watanabe, N.; Ego, T.; Takagaki, K.; Ishiyama, K.; Ohgi, T.; Yano, J. Chemical synthesis of a very long oligoribonucleotide with 2-cyanoethoxymethyl (CEM) as the 2′-O-protecting group: Structural identification and biological activity of a synthetic 110mer precursor-microRNA candidate. Nucleic Acids Res. 2007, 35, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Ohgi, T.; Masutomi, Y.; Ishiyama, K.; Kitagawa, H.; Shiba, Y.; Yano, J. A new RNA synthetic method with a 2′-O-(2-cyanoethoxymethyl) protecting group. Org. Lett. 2005, 7, 3477–3480. [Google Scholar] [CrossRef] [PubMed]
- Usman, N.; Pon, R.T.; Ogilvie, K.K. Preparation of ribonucleoside 3′-O-phosphoramidites and their application to the automated solid phase synthesis of oligonucleotides. Tetrahedron Lett. 1985, 26, 4567–4570. [Google Scholar] [CrossRef]
- Sinha, N.; Davis, P.; Usman, N.; Perez, J.; Hodge, R.; Kremsky, J.; Casale, R. Labile exocyclic amine protection of nucleosides in DNA, RNA and oligonucleotide analog synthesis facililating N-deacylation, minimizing depurination and chain degradation. Biochimie 1993, 75, 13–23. [Google Scholar] [CrossRef]
- Welz, R.; Müller, S. 5-(Benzylmercapto)-1H-tetrazole as activator for 2′-O-TBDMS phosphoramidite building blocks in RNA synthesis. Tetrahedron Lett. 2002, 43, 795–797. [Google Scholar] [CrossRef]
- Westman, E.; Stromberg, R. Removal of t-butyldimethylsilyl protection in RNA-synthesis. Triethylamine trihydrofluoride (TEA, 3HF) is a more reliable alternative to tetrabutylammonium fluoride (TBAF). Nucleic Acids Res. 1994, 22, 2430–2431. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hirota, M.; Waugh, S.M.; Murakami, I.; Suzuki, T.; Muraguchi, M.; Shibamori, M.; Ishikawa, Y.; Jarvis, T.C.; Carter, J.D.; et al. Chemically modified DNA aptamers bind interleukin-6 with high affinity and inhibit signaling by blocking its interaction with interleukin-6 receptor. J. Biol. Chem. 2014, 289, 8706–8719. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.; Sousa, R. Efficient synthesis of nucleic acids heavily modified with non-canonical ribose 2′-groups using a mutantT7 RNA polymerase (RNAP). Nucleic Acids Res. 1999, 27, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjic, N. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef] [PubMed]
- Obika, S.; Nanbu, D.; Hari, Y.; Morio, K.-I.; In, Y.; Ishida, T.; Imanishi, T. Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3, -endo sugar puckering. Tetrahedron Lett. 1997, 38, 8735–8738. [Google Scholar] [CrossRef]
- Koshkin, A.A.; Singh, S.K.; Nielsen, P.; Rajwanshi, V.K.; Kumar, R.; Meldgaard, M.; Olsen, C.E.; Wengel, J. LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 1998, 54, 3607–3630. [Google Scholar] [CrossRef]
- Darfeuille, F.; Hansen, J.B.; Orum, H.; Di Primo, C.; Toulme, J.J. LNA/DNA chimeric oligomers mimic RNA aptamers targeted to the TAR RNA element of HIV-1. Nucleic Acids Res. 2004, 32, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; He, X.; Cui, W.; Wang, K.; Deng, K.; Li, D.; Xu, F. Locked nucleic acid/DNA chimeric aptamer probe for tumor diagnosis with improved serum stability and extended imaging window in vivo. Anal. Chim. Acta 2014, 812, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Wengel, J. Locked vs. unlocked nucleic acids (LNA vs. UNA): Contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 2011, 40, 5680–5689. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.; Hernandez, F.J.; Rasmussen, L.M.; Vester, B.; Wengel, J. Improved thrombin binding aptamer by incorporation of a single unlocked nucleic acid monomer. Nucleic Acids Res. 2011, 39, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Sacca, B.; Lacroix, L.; Mergny, J.L. The effect of chemical modifications on the thermal stability of different G-quadruplex-forming oligonucleotides. Nucleic Acids Res. 2005, 33, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- El-Sagheer, A.H.; Brown, T. Click chemistry with DNA. Chem. Soc. Rev. 2010, 39, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Mutisya, D.; Selvam, C.; Kennedy, S.D.; Rozners, E. Synthesis and properties of triazole-linked RNA. Bioorg. Med. Chem. Lett. 2011, 21, 3420–3422. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.P.; Hrdlicka, P.J. C2′-pyrene-functionalized triazole-linked DNA: Universal DNA/RNA hybridization probes. J. Org. Chem. 2012, 77, 5–16. [Google Scholar] [CrossRef] [PubMed]
- El-Sagheer, A.H.; Brown, T. Click nucleic acid ligation: Applications in biology and nanotechnology. Acc. Chem. Res. 2012, 45, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Srihari, P.; Nagesh, C.; Kiranmai, N.; Nagesh, N.; Idris, M.M. Synthesis of readily accessible triazole-linked dimer deoxynucleoside phosphoramidite for solid-phase oligonucleotide synthesis. Synthesis 2010, 2010, 3710–3714. [Google Scholar] [CrossRef]
- Nuzzi, A.; Massi, A.; Dondoni, A. Model studies toward the synthesis of thymidine oligonucleotides with triazole internucleosidic linkages via iterative Cu(I)-promoted azide–alkyne ligation chemistry. Mol. Inform. 2007, 26, 1191–1199. [Google Scholar]
- Lucas, R.; Zerrouki, R.; Granet, R.; Krausz, P.; Champavier, Y. A rapid efficient microwave-assisted synthesis of a 3′,5′-pentathymidine by copper (I)-catalyzed [3 + 2] cycloaddition. Tetrahedron 2008, 64, 5467–5471. [Google Scholar] [CrossRef]
- Varizhuk, A.M.; Kaluzhny, D.N.; Novikov, R.A.; Chizhov, A.O.; Smirnov, I.P.; Chuvilin, A.N.; Tatarinova, O.N.; Fisunov, G.Y.; Pozmogova, G.E.; Florentiev, V.L. Synthesis of triazole-linked oligonucleotides with high affinity to DNA complements and an analysis of their compatibility with biosystems. J. Org. Chem. 2013, 78, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Varizhuk, A.M.; Tsvetkov, V.B.; Tatarinova, O.N.; Kaluzhny, D.N.; Florentiev, V.L.; Timofeev, E.N.; Shchyolkina, A.K.; Borisova, O.F.; Smirnov, I.P.; Grokhovsky, S.L.; et al. Synthesis, characterization and in vitro activity of thrombin-binding DNA aptamers with triazole internucleotide linkages. Eur. J. Med. Chem. 2013, 67, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Wlotzka, B.; Leva, S.; Eschgfaller, B.; Burmeister, J.; Kleinjung, F.; Kaduk, C.; Muhn, P.; Hess-Stumpp, H.; Klussmann, S. In vivo properties of an anti-GnRH Spiegelmer: An example of an oligonucleotide-based therapeutic substance class. Proc. Natl. Acad. Sci. USA 2002, 99, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Leva, S.; Lichte, A.; Burmeister, J.; Muhn, P.; Jahnke, B.; Fesser, D.; Erfurth, J.; Burgstaller, P.; Klussmann, S. GnRH binding RNA and DNA Spiegelmers: A novel approach toward GnRH antagonism. Chem. Biol. 2002, 9, 351–359. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, C.; Lv, Q.; Li, D.; Xu, X.; Liu, B.; Lu, A.; Zhang, G. Molecular selection, modification and development of therapeutic oligonucleotide aptamers. Int. J. Mol. Sci. 2016, 17, 358. [Google Scholar] [CrossRef] [PubMed]
- Purschke, W.G.; Radtke, F.; Kleinjung, F.; Klussmann, S. A DNA Spiegelmer to staphylococcal enterotoxin B. Nucleic Acids Res. 2003, 31, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.H.; Kim, J.H.; Noh, Y.H.; Noh, G.J.; Lee, S.W. Pharmacokinetics of a cholesterol-conjugated aptamer against the Hepatitis C Virus (HCV) NS5B Protein. Mol. Ther. Nucleic Acids 2015, 4, e254. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.C.; Collins, B.D.; Zhang, T.; Green, L.S.; Sebesta, D.P.; Bell, C.; Kellogg, E.; Gill, S.C.; Magallanez, A.; Knauer, S.; et al. Liposome-anchored vascular endothelial growth factor aptamers. Bioconjug. Chem. 1998, 9, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Green, L.S.; Jellinek, D.; Bell, C.; Beebe, L.A.; Feistner, B.D.; Gill, S.C.; Jucker, F.M.; Janjic, N. Nuclease-resistant nucleic acid ligands to vascular permeability factor/vascular endothelial growth factor. Chem. Biol. 1995, 2, 683–695. [Google Scholar] [CrossRef]
- Hoffmann, S.; Hoos, J.; Klussmann, S.; Vonhoff, S. RNA aptamers and spiegelmers: Synthesis, purification, and post-synthetic PEG conjugation. Curr. Protoc. Nucleic Acid Chem. 2011, 46, 1–30. [Google Scholar]
- Da Pieve, C.; Blackshaw, E.; Missailidis, S.; Perkins, A.C. PEGylation and biodistribution of an anti-MUC1 aptamer in MCF-7 tumor-bearing mice. Bioconjug. Chem. 2012, 23, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Abdul-Wahid, A.; Fischer, N.W.; Huang, E.H.; Cydzik, M.; Gariépy, J. Targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as a novel therapeutic strategy for the treatment of disseminated cancers. Mol. Ther. Nucleic Acids 2015, 4, e237. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Neoh, K.G.; Kang, E.T.; Choe, W.S.; Su, X. PEGylated anti-MUC1 aptamer-doxorubicin complex for targeted drug delivery to MCF7 breast cancer cells. Macromol. Biosci. 2011, 11, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Teng, Y.; Bates, P.J. A new paradigm for aptamer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010, 70, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.L.; Zhu, G.; Xiao, X.; Puszyk, W.; Sefah, K.; Wu, Q.; Tan, W.; Liu, C. A synthetic aptamer-drug adduct for targeted liver cancer therapy. PLoS ONE 2015, 10, e0136673. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, M.; Yamashige, R.; Matsunaga, K.I.; Yokoyama, S.; Hirao, I. Generation of high-affinity DNA aptamers using an expanded genetic alphabet. Nat. Biotechnol. 2013, 31, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Yang, Z.; Bradley, K.M.; Hoshika, S.; Jimenez, E.; Zhang, L.; Zhu, G.; Shanker, S.; Yu, F.; Turek, D.; et al. In vitro selection with artificial expanded genetic information systems. Proc. Natl. Acad. Sci. USA 2014, 111, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Durante, M.; Glushakova, L.G.; Sharma, N.; Leal, N.A.; Bradley, K.M.; Chen, F.; Benner, S.A. Conversion strategy using an expanded genetic alphabet to assay nucleic acids. Anal. Chem. 2013, 85, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, A.; Yuhina, E.; Frolova, E.; Kravchenko, J.; Chumakov, S. Expanding the application potential of DNA aptamers by their functionalization. Russ. J. Bioorg. Chem. 2016, 42, 1–13. [Google Scholar] [CrossRef]
- Tonkinson, J.L.; Guvakova, M.; Khaled, Z.; Lee, J.; Yakubov, L.; Marshall, W.S.; Caruthers, M.H.; Stein, C. Cellular pharmacology and protein binding of phosphoromonothioate and phosphorodithioate oligodeoxynucleotides: A comparative study. Antisense Res. Dev. 1994, 4, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zandarashvili, L.; Nguyen, D.; Anderson, K.M.; White, M.A.; Gorenstein, D.G.; Iwahara, J. Entropic enhancement of protein-DNA affinity by oxygen-to-sulfur substitution in DNA phosphate. Biophys. J. 2015, 109, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Vaught, J.D.; Bock, C.; Carter, J.; Fitzwater, T.; Otis, M.; Schneider, D.; Rolando, J.; Waugh, S.; Wilcox, S.K.; Eaton, B.E. Expanding the chemistry of DNA for in vitro selection. J. Am. Chem. Soc. 2010, 132, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R.; Gelinas, A.D.; Zhang, C.; Rohloff, J.C.; Carter, J.D.; O’Connell, D.; Waugh, S.M.; Wolk, S.K.; Mayfield, W.S.; Burgin, A.B.; et al. Unique motifs and hydrophobic interactions shape the binding of modified DNA ligands to protein targets. Proc. Natl. Acad. Sci. USA 2012, 109, 19971–19976. [Google Scholar] [CrossRef] [PubMed]
- Rohloff, J.C.; Gelinas, A.D.; Jarvis, T.C.; Ochsner, U.A.; Schneider, D.J.; Gold, L.; Janjic, N. Nucleic acid ligands with protein-like side chains: Modified aptamers and their use as diagnostic and therapeutic agents. Mol. Ther. Nucleic Acids 2014, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Brauer, A.B.; Brode, S.; Schmidt, K.S.; Perbandt, M.; Meyer, A.; Rypniewski, W.; Betzel, C.; Kurreck, J.; Furste, J.P.; et al. Comparative crystallization and preliminary X-ray diffraction studies of locked nucleic acid and RNA stems of a tenascin C-binding aptamer. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62 Pt 7, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Oberthuer, D.; Gao, J.; Eichert, A.; Quast, F.G.; Betzel, C.; Nitsche, A.; Erdmann, V.A.; Furste, J.P. Crystallization and preliminary X-ray diffraction data of an LNA 7-mer duplex derived from a ricin aptamer. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65 Pt 9, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, A.D.; Davies, D.R.; Edwards, T.E.; Rohloff, J.C.; Carter, J.D.; Zhang, C.; Gupta, S.; Ishikawa, Y.; Hirota, M.; Nakaishi, Y.; et al. Crystal structure of interleukin-6 in complex with a modified nucleic acid ligand. J. Biol. Chem. 2014, 289, 8720–8734. [Google Scholar] [CrossRef] [PubMed]
- Hottin, A.; Marx, A. Structural insights into the processing of nucleobase-modified nucleotides by DNA polymerases. Acc. Chem. Res. 2016, 49, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Umezu-Goto, M.; Kishi, Y.; Taira, A.; Hama, K.; Dohmae, N.; Takio, K.; Yamori, T.; Mills, G.B.; Inoue, K.; Aoki, J. Autotaxin has lysophospholipase d activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002, 158, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase d, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ikeda, H.; Miyakawa, S.; Futakawa, S.; Nonaka, Y.; Fujiwara, M.; Okudaira, S.; Kano, K.; Aoki, J.; Morita, J.; et al. Structural basis for specific inhibition of autotaxin by a DNA aptamer. Nat. Struct. Mol. Biol. 2016, 23, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Kamtekar, S.; Christodoulou, E.; Day, J.E.; Wu, T.; Fulkerson, Z.; Albers, H.M.; van Meeteren, L.A.; Houben, A.J.; van Zeijl, L.; et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 2011, 18, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Okabe, T.; Okudaira, S.; Nishimasu, H.; Ishitani, R.; Kojima, H.; Nureki, O.; Aoki, J.; Nagano, T. Screening and X-ray crystal structure-based optimization of autotaxin (ENPP2) inhibitors, using a newly developed fluorescence probe. ACS Chem. Biol. 2013, 8, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Duchardt-Ferner, E.; Juen, M.; Kreutz, C.; Wöhnert, J. NMR resonance assignments for the tetramethylrhodamine binding RNA aptamer 3 in complex with the ligand 5-carboxy-tetramethylrhodamine. Biomol. NMR Assign. 2017, 11, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Amano, R.; Aoki, K.; Miyakawa, S.; Nakamura, Y.; Kozu, T.; Kawai, G.; Sakamoto, T. NMR monitoring of the SELEX process to confirm enrichment of structured RNA. Sci. Rep. 2017, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Onodera, K.; Fujita, H.; Sakamoto, T.; Akitomi, J.; Kaneko, N.; Shiratori, I.; Kuwahara, M.; Horii, K.; Waga, I. Selection characterization and application of artificial DNA aptamer containing appended bases with sub-nanomolar affinity for a salivary biomarker. Sci. Rep. 2017, 7, 42716. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, D.; Chen, T.; Liu, Z.; Hongdilokkul, N.; Romesberg, F.E. Selection of 2′-fluoro-modified aptamers with optimized properties. J. Am. Chem. Soc. 2017, 139, 2892–2895. [Google Scholar] [CrossRef] [PubMed]

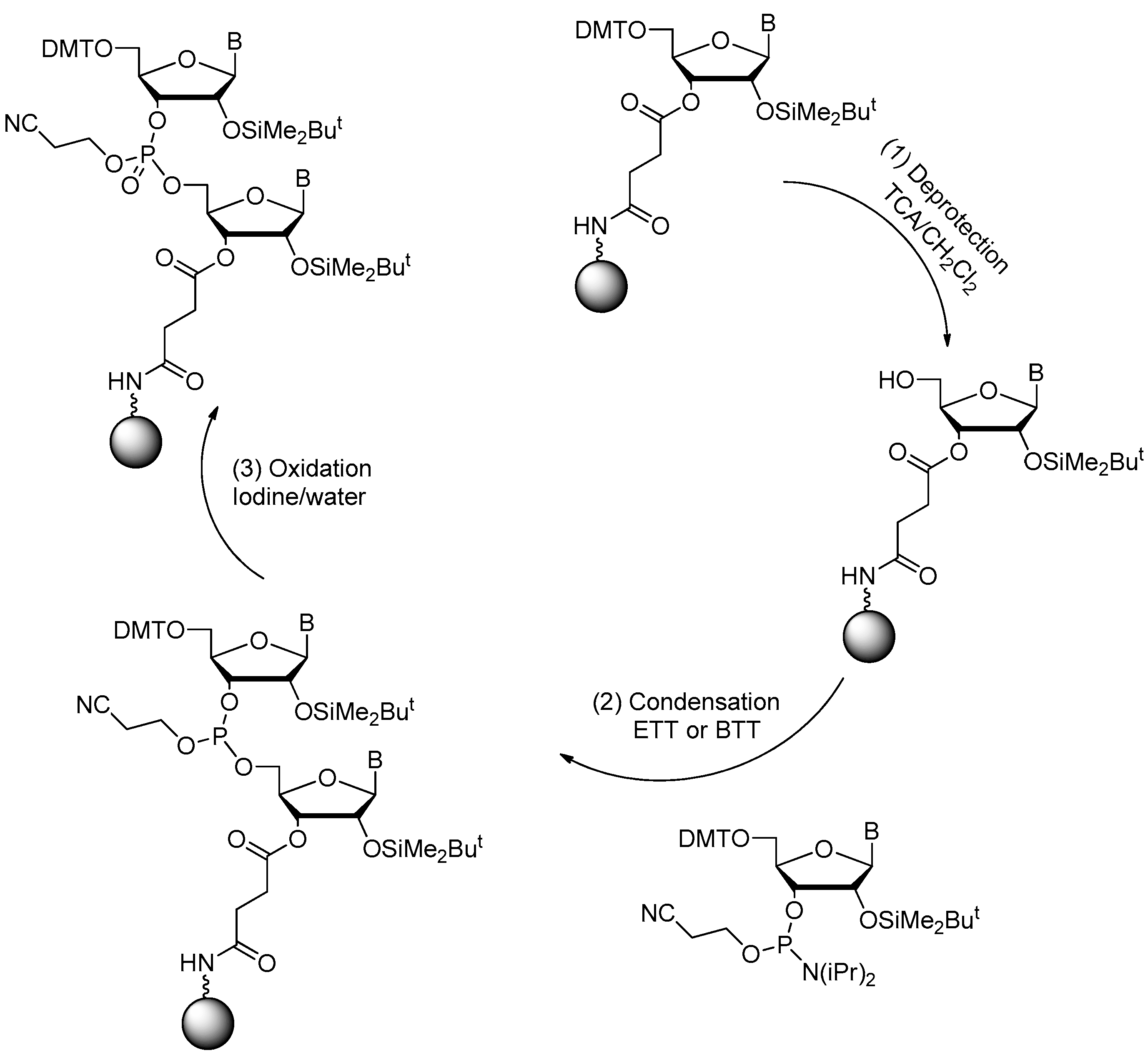

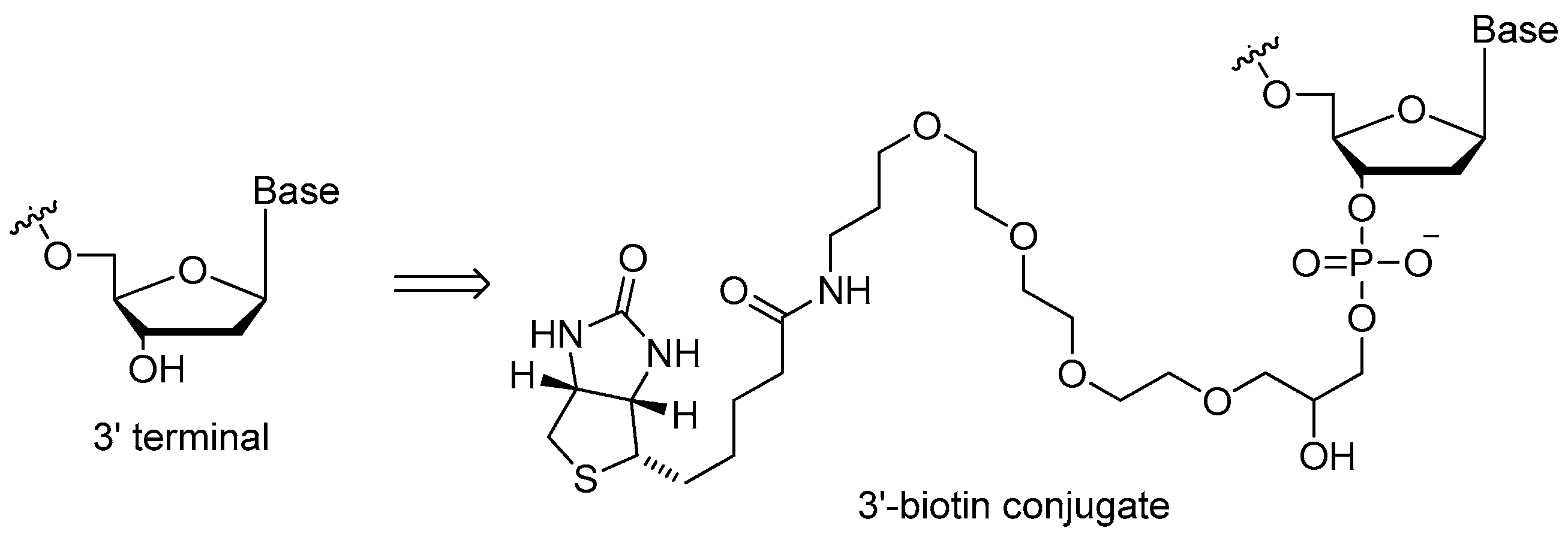





| Modification Sites | Strategy | Applications |
|---|---|---|
| ends of nucleic acid chain | terminal 3′–3′or 5′–5′internucleotide linkage1, 3′-biotin conjugates; | [12,15,36,47] |
| sugar ring of nucleoside | 2′-fluoro, 2′-O-methyl and 2′-amino-substitutions 1, locked nucleic acid (LNA), unlocked nucleic acid (UNA) and 2′-deoxy-2′-fluoro-d-arabinonucleic acid (2′-F ANA); | [25,48,49,52,53,54,55] |
| phosphodiester linkage | methylphosphonate or phosphorothioate, replaced by triazole; | [23,24,56,57,58,59] |
| mirror image | l-enantiomeric oligonucleotide aptamers (Spiegelmers) | [66,67,68,69,70] |
| Modification Sites | Strategy | Applications |
|---|---|---|
| ends of nucleic acid chain | 5′-end with cholesterol; 5′-end with dialkyl lipids; 5′-end PEGylation 1 | [32,33,71,72,73,74,75,76,77] |
| Modification Sites | Strategy | Applications |
|---|---|---|
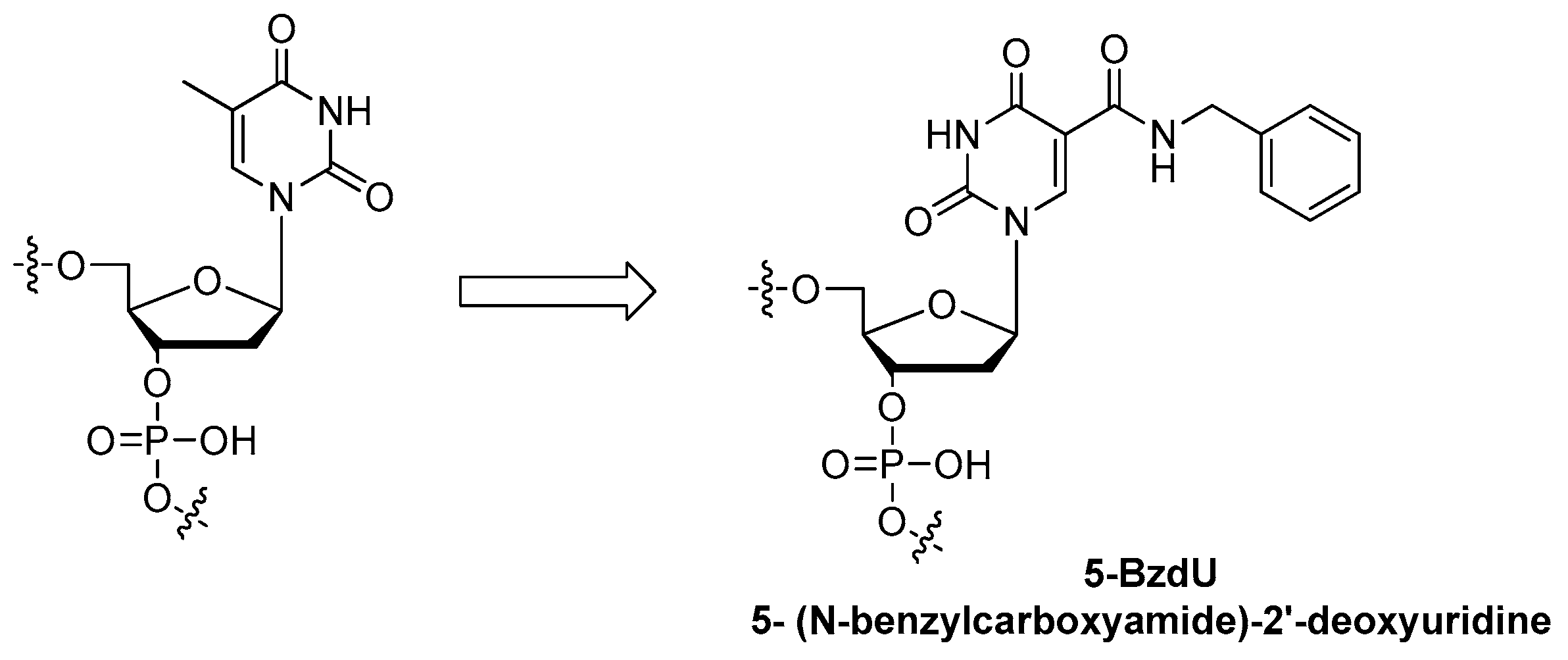
| base of nucleoside | 5-(N-benzylcarboxyamide)-2′-deoxyuridine modification 1, Slow Off-rate Modified Aptamers (SOMAmers) | [78,79,80,81,82,83,84] |
| phosphodiester linkage | phosphorodithioate (PS2) substitution | [10,85,86] |
| Strategy | Nuclease Resistance | Improving Binding Affinity and Target Selectivity | Resistance to Renal Clearance |
|---|---|---|---|
| 3′-3′inversion/ 3′-T capping | [12,20,21] | ||
| 5′-5′inversion | [12] | ||
| 3′-biotin conjugates | [20,36] | ||
| 2′-fluoro, 2′-O-methyl and 2′-amino-substitutions1 | [39,48,49] | ||
| locked nucleic acid (LNA) | [52,53] | ||
| unlocked nucleic acid (UNA) | [54,55] | ||
| 2′-deoxy-2′-fluoro-d-arabinonucleic acid (2′-F ANA) | [25] | ||
| methylphosphonate | [56] | ||
| phosphorothioate | [23,24] | ||
| replaced by triazole | [57,58,59,60] | ||
| l-enantiomeric oligonucleotide aptamers (Spiegelmers) | [66,67,68,69,70] | ||
| 5′-end with cholesterol | [32,33,71] | ||
| 5′-end with dialkyl lipids | [72,73] | ||
| 5′-end PEGylation | [32,74,75,76,77] | ||
| 5-(N-benzylcarboxyamide)-2-deoxyuridine modification1, Slow Off-rate Modified Aptamers (SOMAmers) | [78,79,80,81,82,83,84] | ||
| phosphorodithioate (PS2) substitution | [10,85,86] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, S.; Yao, H.; Wang, L.; Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes. Int. J. Mol. Sci. 2017, 18, 1683. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081683
Ni S, Yao H, Wang L, Lu J, Jiang F, Lu A, Zhang G. Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes. International Journal of Molecular Sciences. 2017; 18(8):1683. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081683
Chicago/Turabian StyleNi, Shuaijian, Houzong Yao, Lili Wang, Jun Lu, Feng Jiang, Aiping Lu, and Ge Zhang. 2017. "Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes" International Journal of Molecular Sciences 18, no. 8: 1683. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081683