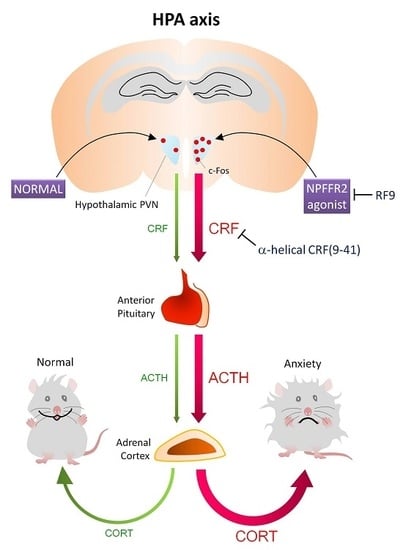
NPFFR2 Activates the HPA Axis and Induces Anxiogenic Effects in Rodents
Abstract
:1. Introduction
2. Results
2.1. Activation of NPFFR2 Stimulates the HPA Axis in Mice and Rats
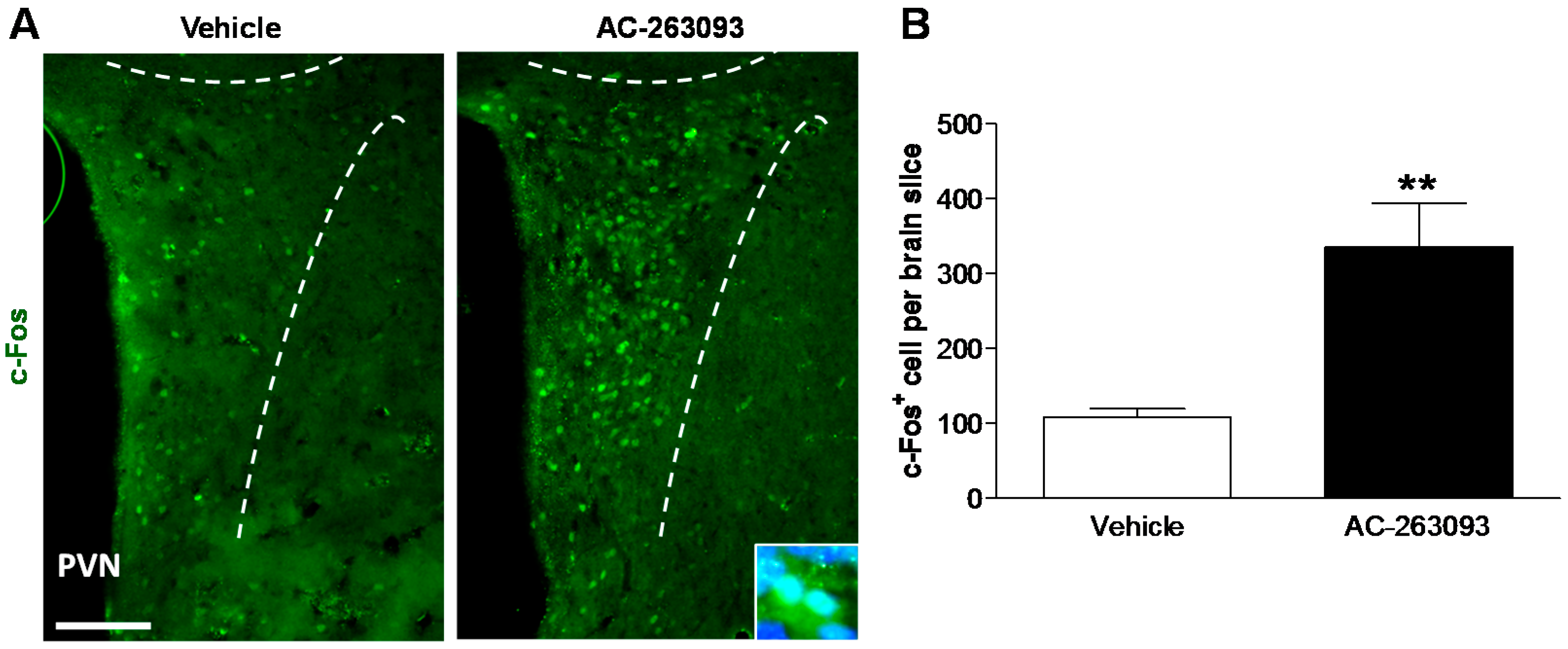
2.2. NPFFR2 Agonist Induces Activity of Hypothalamic PVN Neurons in Mice
2.3. NPFFR2 Stimulation Induces Anxiety-Like Behavior
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. NPFFR2 Agonists
4.3. Implantation and Cannulation in the Lateral Ventricle
4.4. Pharmacological Intervention Studies
4.5. Immunohistochemistry (IHC)
4.6. CORT ELISA Assay
4.7. Elevated Plus Maze (EPM)
4.8. Open Field Activity
4.9. Statistical Analysis
4.10. Experimental Summary
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Price, D.A.; Greenberg, M.J. Structure of a molluscan cardioexcitatory neuropeptide. Science 1977, 197, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Mulderry, P.K.; Ghatei, M.A.; Bishop, A.E.; Allen, Y.S.; Polak, J.M.; Bloom, S.R. Distribution and chromatographic characterisation of CGRP-like immunoreactivity in the brain and gut of the rat. Regul. Pept. 1985, 12, 133–143. [Google Scholar] [CrossRef]
- Yang, H.Y.; Tao, T.; Iadarola, M.J. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides 2008, 42, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W.; Lakhlani, P.P.; et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000, 275, 39324–39331. [Google Scholar] [CrossRef] [PubMed]
- Elshourbagy, N.A.; Ames, R.S.; Fitzgerald, L.R.; Foley, J.J.; Chambers, J.K.; Szekeres, P.G.; Evans, N.A.; Schmidt, D.B.; Buckley, P.T.; Dytko, G.M.; et al. Receptor for the pain modulatory neuropeptides FF and AF is an orphan G protein-coupled receptor. J. Biol. Chem. 2000, 275, 25965–25971. [Google Scholar] [CrossRef] [PubMed]
- Hinuma, S.; Shintani, Y.; Fukusumi, S.; Iijima, N.; Matsumoto, Y.; Hosoya, M.; Fujii, R.; Watanabe, T.; Kikuchi, K.; Terao, Y.; et al. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat. Cell Biol. 2000, 2, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Gouarderes, C.; Quelven, I.; Mollereau, C.; Mazarguil, H.; Rice, S.Q.; Zajac, J.M. Quantitative autoradiographic distribution of NPFF1 neuropeptide FF receptor in the rat brain and comparison with NPFF2 receptor by using [125I]YVP and [125I]EYF as selective radioligands. Neuroscience 2002, 115, 349–361. [Google Scholar] [CrossRef]
- Gouarderes, C.; Puget, A.; Zajac, J.M. Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: A comparative autoradiographic study. Synapse 2004, 51, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Ayachi, S.; Simonin, F. Involvement of mammalian RF-amide peptides and their receptors in the modulation of nociception in rodents. Front. Endocrinol. 2014, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, M.; Rathmann, D.; Beck-Sickinger, A.G. RFamide peptides: Structure, function, mechanisms and pharmaceutical potential. Pharmaceuticals 2011, 4, 1248–1280. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Goncharuk, V. Role of neuropeptide FF in central cardiovascular and neuroendocrine regulation. Front. Endocrinol. 2013, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, X.M.; Martin, W.J.; McDonald, T.P.; Clements, M.K.; Jiang, Q.; Zeng, Z.; Jacobson, M.; Williams, D.L., Jr.; Yu, H.; et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J. Biol. Chem. 2001, 276, 36961–36969. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Kalso, E.; Nieminen, M.; Kontinen, V.K.; Brandt, A.; Pertovaara, A. Neuropeptide FF and modulation of pain. Brain Res. 1999, 848, 191–196. [Google Scholar] [CrossRef]
- Mouledous, L.; Mollereau, C.; Zajac, J.M. Opioid-modulating properties of the neuropeptide FF system. Biofactors 2010, 36, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G.A.; van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Herman, J.P.; Tasker, J.G. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front. Endocrinol. 2016, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF family of neuropeptides and their receptors-mediators of the central stress response. Curr. Mol. Pharmacol. 2017. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Simonin, F.; Bourguignon, J.J.; Harris, K.H. Neuropeptide FF and neuropeptide VF inhibit GABAergic neurotransmission in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1872–R1880. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; MacTavish, D.; Harris, K.H. Neuropeptide FF (NPFF) control of magnocellular neurosecretory cells of the rat hypothalamic paraventricular nucleus (PVN). Peptides 2006, 27, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Jaszberenyi, M.; Bagosi, Z.; Thurzo, B.; Foldesi, I.; Szabo, G.; Telegdy, G. Endocrine, behavioral and autonomic effects of neuropeptide AF. Horm. Behav. 2009, 56, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Csabafi, K.; Jaszberenyi, M.; Bagosi, Z.; Liptak, N.; Telegdy, G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav. Brain Res. 2013, 241, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Jaszberenyi, M.; Bagosi, Z.; Csabafi, K.; Palotai, M.; Telegdy, G. The actions of neuropeptide SF on the hypothalamic-pituitary-adrenal axis and behavior in rats. Regul. Pept. 2014, 188, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Brownjohn, P.W.; Dyer, B.S.; Beltramo, M.; Walker, C.S.; Hay, D.L.; Painter, G.F.; Tyndall, J.D.; Anderson, G.M. Anxiogenic and stressor effects of the hypothalamic neuropeptide RFRP-3 are overcome by the NPFFR antagonist GJ14. Endocrinology 2015, 156, 4152–4162. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Liu, T.Y.; Yang, C.Y.; Yu, Y.L.; Chen, T.C.; Day, Y.J.; Chang, C.C.; Huang, G.J.; Chen, J.C. Chronic activation of NPFFR2 stimulates the stress-related depressive behaviors through HPA axis modulation. Psychoneuroendocrinology 2016, 71, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, G.; Bertozzi, F.; Kelly, N.M.; Pawlas, J.; Scully, A.L.; Nash, N.R.; Gardell, L.R.; Lameh, J.; Olsson, R. Discovery of selective nonpeptidergic neuropeptide FF2 receptor agonists. J. Med. Chem. 2009, 52, 6511–6514. [Google Scholar] [CrossRef] [PubMed]
- Fukusumi, S.; Fujii, R.; Hinuma, S. Recent advances in mammalian RFamide peptides: The discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides 2006, 27, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Iadarola, M.J. Modulatory roles of the NPFF system in pain mechanisms at the spinal level. Peptides 2006, 27, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Simonin, F.; Schmitt, M.; Laulin, J.P.; Laboureyras, E.; Jhamandas, J.H.; MacTavish, D.; Matifas, A.; Mollereau, C.; Laurent, P.; Parmentier, M.; et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. USA 2006, 103, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, Y.Q.; He, F.; Guo, J.; Guo, J.; Chen, Q.; Wang, R. Inhibition of neuropeptide FF (NPFF)-induced hypothermia and anti-morphine analgesia by RF9, a new selective NPFF receptors antagonist. Regul. Pept. 2008, 147, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wang, S.B.; Ma, J.L.; Guo, J.; Fang, Q.; Sun, T.; Zhuang, Y.; Wang, R. Neuropeptide FF receptor antagonist, RF9, attenuates the fever induced by central injection of LPS in mice. Peptides 2011, 32, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Maletinska, L.; Ticha, A.; Nagelova, V.; Spolcova, A.; Blechova, M.; Elbert, T.; Zelezna, B. Neuropeptide FF analog RF9 is not an antagonist of NPFF receptor and decreases food intake in mice after its central and peripheral administration. Brain Res. 2013, 1498, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Leon, S.; Li, H.; Pinilla, L.; Carroll, R.S.; Tena-Sempere, M.; Kaiser, U.B. RF9 Acts as a KISS1R Agonist In Vivo and In Vitro. Endocrinology 2015, 156, 4639–4648. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.; Canpolat, S.; Ozcan, M.; Ozgocer, T.; Kelestimur, H. Kisspeptin antagonist prevents RF9-induced reproductive changes in female rats. Reproduction 2015, 149, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; MacTavish, D. Central administration of neuropeptide FF causes activation of oxytocin paraventricular hypothalamic neurones that project to the brainstem. J. Neuroendocrinol. 2003, 15, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.W.; Sawchenko, P.E.; Lind, R.W. Regulation of multiple peptides in CRF parvocellular neurosecretory neurons: Implications for the stress response. Prog. Brain Res. 1986, 68, 169–190. [Google Scholar] [PubMed]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.A.; Alpar, A.; Zhang, M.D.; Zeisel, A.; Calas, A.; Landry, M.; Fuszard, M.; Shirran, S.L.; Schnell, R.; Dobolyi, A.; et al. A secretagogin locus of the mammalian hypothalamus controls stress hormone release. EMBO J. 2015, 34, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Mochiduki, A.; Sugimoto, Y.; Suzuki, Y.; Itoi, K.; Inoue, K. Prolactin-releasing peptide regulates the cardiovascular system via corticotrophin-releasing hormone. J. Neuroendocrinol. 2009, 21, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, M.; Brandt, A.; Wurster, S.; Savola, J.M.; Panula, P. Prolactin releasing peptide has high affinity and efficacy at neuropeptide FF2 receptors. J. Pharmacol. Exp. Ther. 2003, 305, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; MacTavish, D.; Simonin, F.; Bourguignon, J.J.; Watanabe, T.; Jhamandas, J.H. Prolactin-releasing peptide effects in the rat brain are mediated through the Neuropeptide FF receptor. Eur. J. Neurosci. 2009, 30, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Nakahiro, M.; Arakawa, O.; Narahashi, T.; Ukai, S.; Kato, Y.; Nishinuma, K.; Nishimura, T. Dimethyl sulfoxide (DMSO) blocks GABA-induced current in rat dorsal root ganglion neurons. Neurosci. Lett. 1992, 138, 5–8. [Google Scholar] [CrossRef]
- Nasrallah, F.A.; Garner, B.; Ball, G.E.; Rae, C. Modulation of brain metabolism by very low concentrations of the commonly used drug delivery vehicle dimethyl sulfoxide (DMSO). J. Neurosci. Res. 2008, 86, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Mohammadi, E.; Allahtavakoli, M.; Shamsizadeh, A.; Roohbakhsh, A.; Haghparast, A. Effects of dimethyl sulfoxide on neuronal response characteristics in deep layers of rat barrel cortex. Basic Clin. Neurosci. 2016, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Sato, M. The effect of dimethyl sulfoxide on the neuronal excitability and cholinergic transmission in Aplysia ganglion cells. Ann. N. Y. Acad. Sci. 1975, 243, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.H.; Gibula-Bruzda, E.; Koltunowska, D.; Raoof, H.; Suder, P.; Silberring, J. Modulation of neuropeptide FF (NPFF) receptors influences the expression of amphetamine-induced conditioned place preference and amphetamine withdrawal anxiety-like behavior in rats. Peptides 2012, 33, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.; Pachuta, A.; Bochenski, M.; Silberring, J. Dansyl-PQRamide, a putative antagonist of NPFF receptors, reduces anxiety-like behavior of ethanol withdrawal in a plus-maze test in rats. Peptides 2009, 30, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Cador, M.; Marco, N.; Stinus, L.; Simonnet, G. Interaction between neuropeptide FF and opioids in the ventral tegmental area in the behavioral response to novelty. Neuroscience 2002, 110, 309–318. [Google Scholar] [CrossRef]
- Lin, Y.T.; Kao, S.C.; Day, Y.J.; Chang, C.C.; Chen, J.C. Altered nociception and morphine tolerance in neuropeptide FF receptor type 2 over-expressing mice. Eur. J. Pain. 2016, 20, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Watson, S.J. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Paxinos, G., Watson, C., Eds.; Academic Press: Cambridge, MA, USA, 1986; 264p, ISBN 0125476213. [Google Scholar]
- Gaszner, B.; Kormos, V.; Kozicz, T.; Hashimoto, H.; Reglodi, D.; Helyes, Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience 2012, 202, 283–299. [Google Scholar] [PubMed]
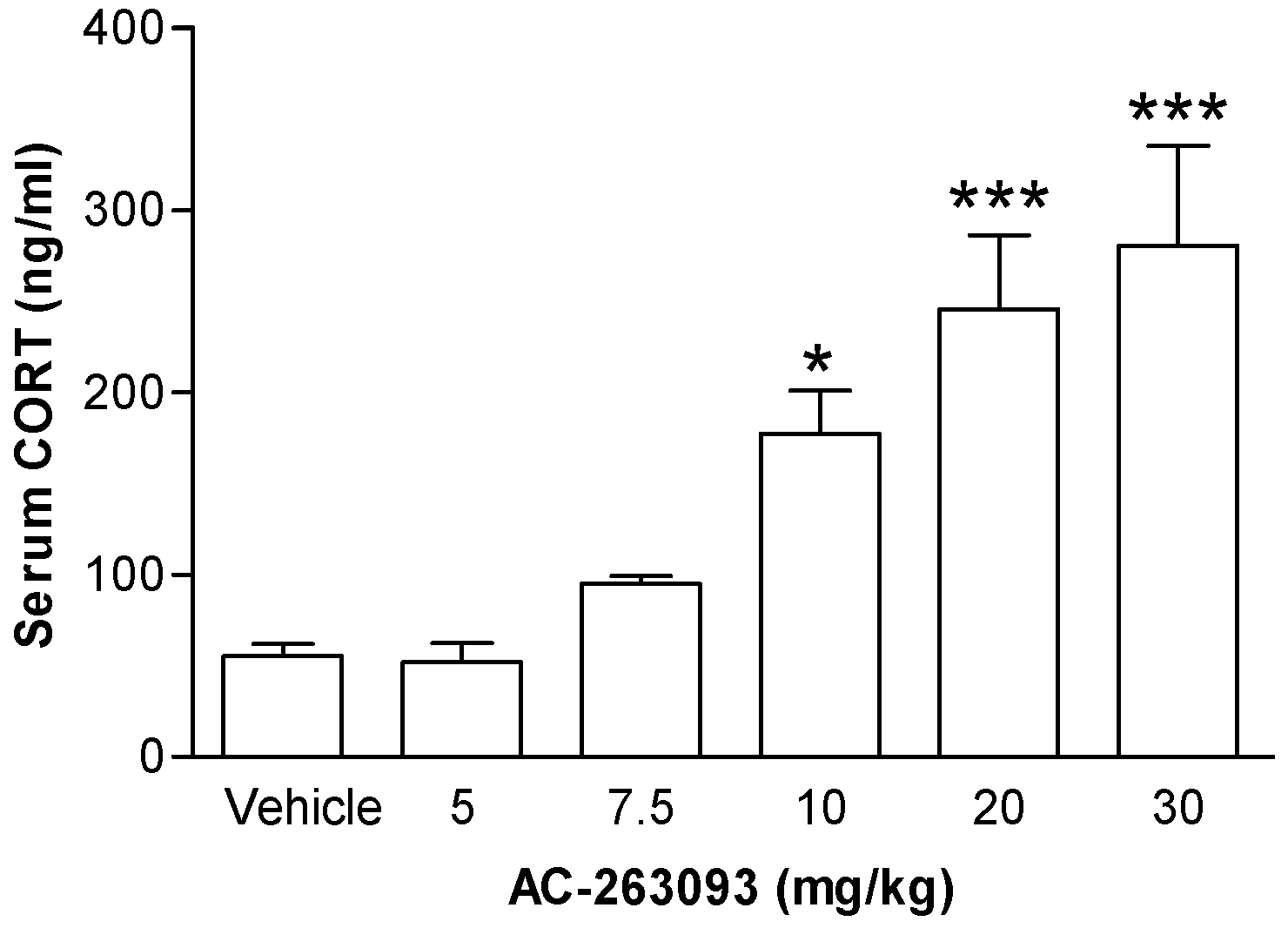
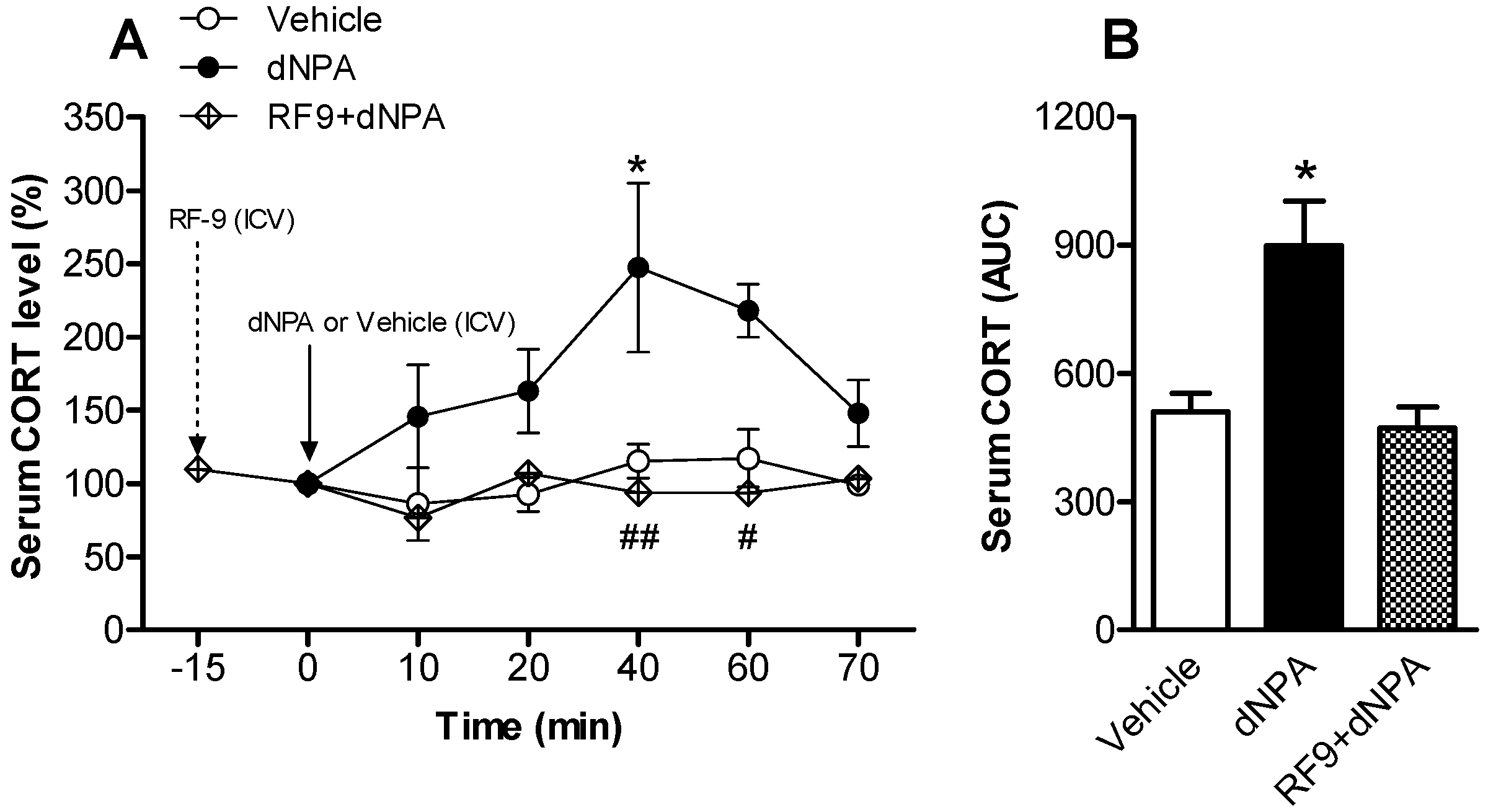
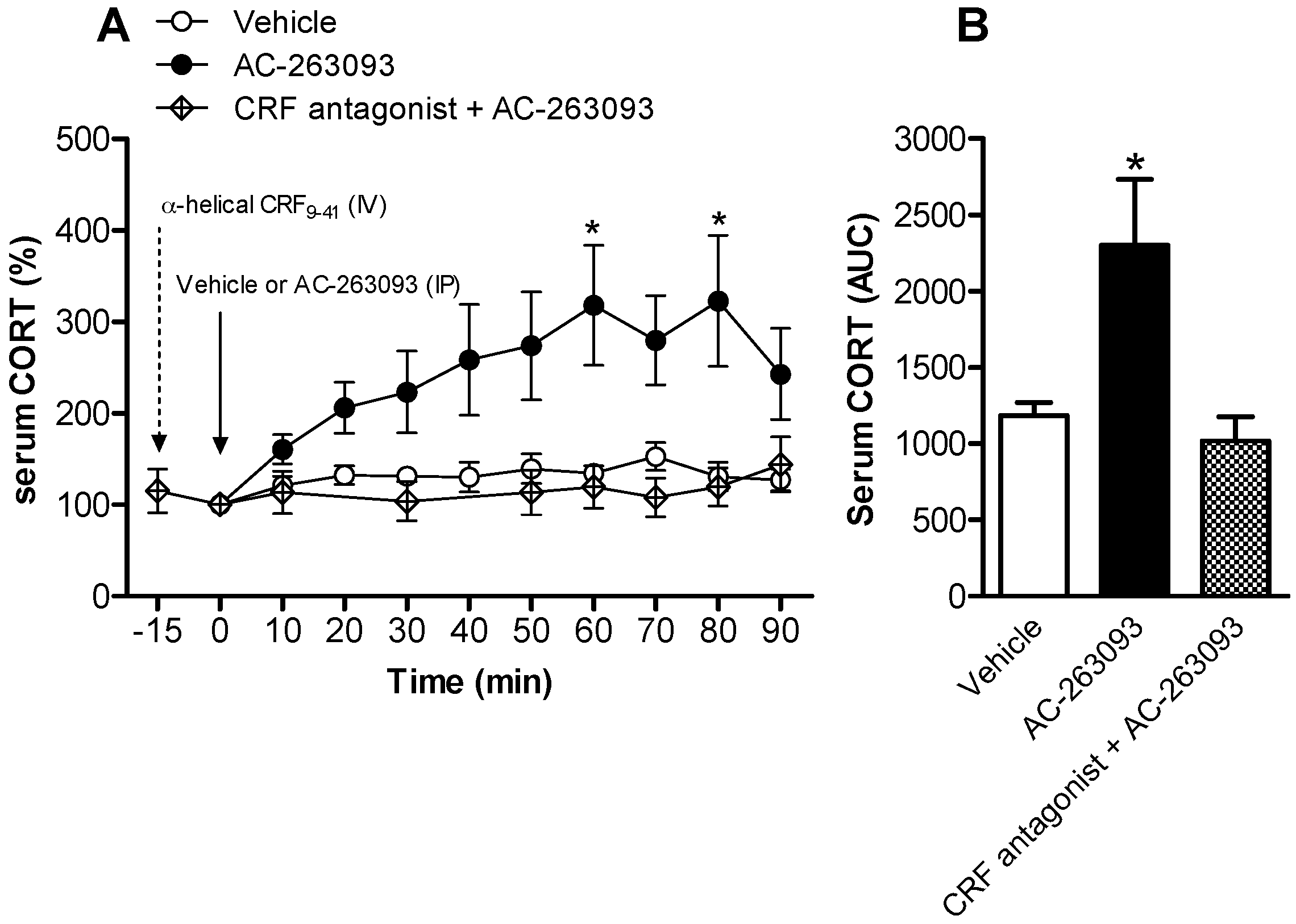

| Experiment in Figure | Subject | Experimental Groups | n Numbers |
|---|---|---|---|
| Figure 1 | Mouse | Vehicle | 5 |
| AC-263093 | 5 | ||
| Figure 2 | Rat | Vehicle | 5 |
| dNPA | 7 | ||
| RF9 + dNPA | 4 | ||
| RF9 (Figure S2) | 6 | ||
| Figure 3 | Rat | Vehicle | 7 |
| AC-263093 | 11 | ||
| α-helical CRF(9-41) + AC-263093 | 4 | ||
| α-helical CRF(9-41) (Figure S2) | 6 | ||
| Figure 4 | Mouse | Vehicle | 4 |
| AC-263093 | 4 | ||
| Figure 5 | Mouse | Vehicle | 7 |
| AC-263093 | 8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Yu, Y.-L.; Hong, W.-C.; Yeh, T.-S.; Chen, T.-C.; Chen, J.-C. NPFFR2 Activates the HPA Axis and Induces Anxiogenic Effects in Rodents. Int. J. Mol. Sci. 2017, 18, 1810. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081810
Lin Y-T, Yu Y-L, Hong W-C, Yeh T-S, Chen T-C, Chen J-C. NPFFR2 Activates the HPA Axis and Induces Anxiogenic Effects in Rodents. International Journal of Molecular Sciences. 2017; 18(8):1810. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081810
Chicago/Turabian StyleLin, Ya-Tin, Yu-Lian Yu, Wei-Chen Hong, Ting-Shiuan Yeh, Ting-Chun Chen, and Jin-Chung Chen. 2017. "NPFFR2 Activates the HPA Axis and Induces Anxiogenic Effects in Rodents" International Journal of Molecular Sciences 18, no. 8: 1810. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18081810