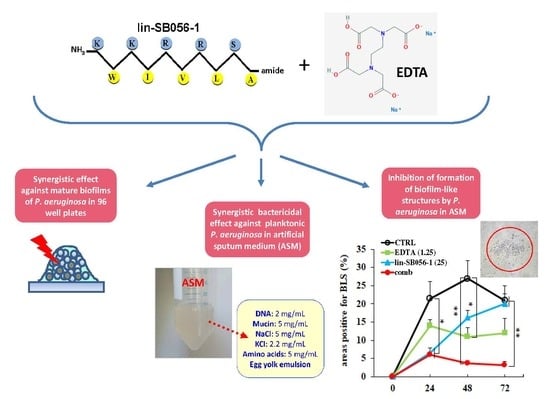
The Semi-Synthetic Peptide Lin-SB056-1 in Combination with EDTA Exerts Strong Antimicrobial and Antibiofilm Activity against Pseudomonas aeruginosa in Conditions Mimicking Cystic Fibrosis Sputum
Abstract
:1. Introduction
2. Results
2.1. Bactericidal Activity and Killing Kinetics of Lin-SB056-1 against Planktonic Cells of Mucoid and Non-Mucoid Strains of P. aeruginosa, in Sodium Phosphate Buffer (SPB) with 1% Tryptone Soya Broth (TSB)
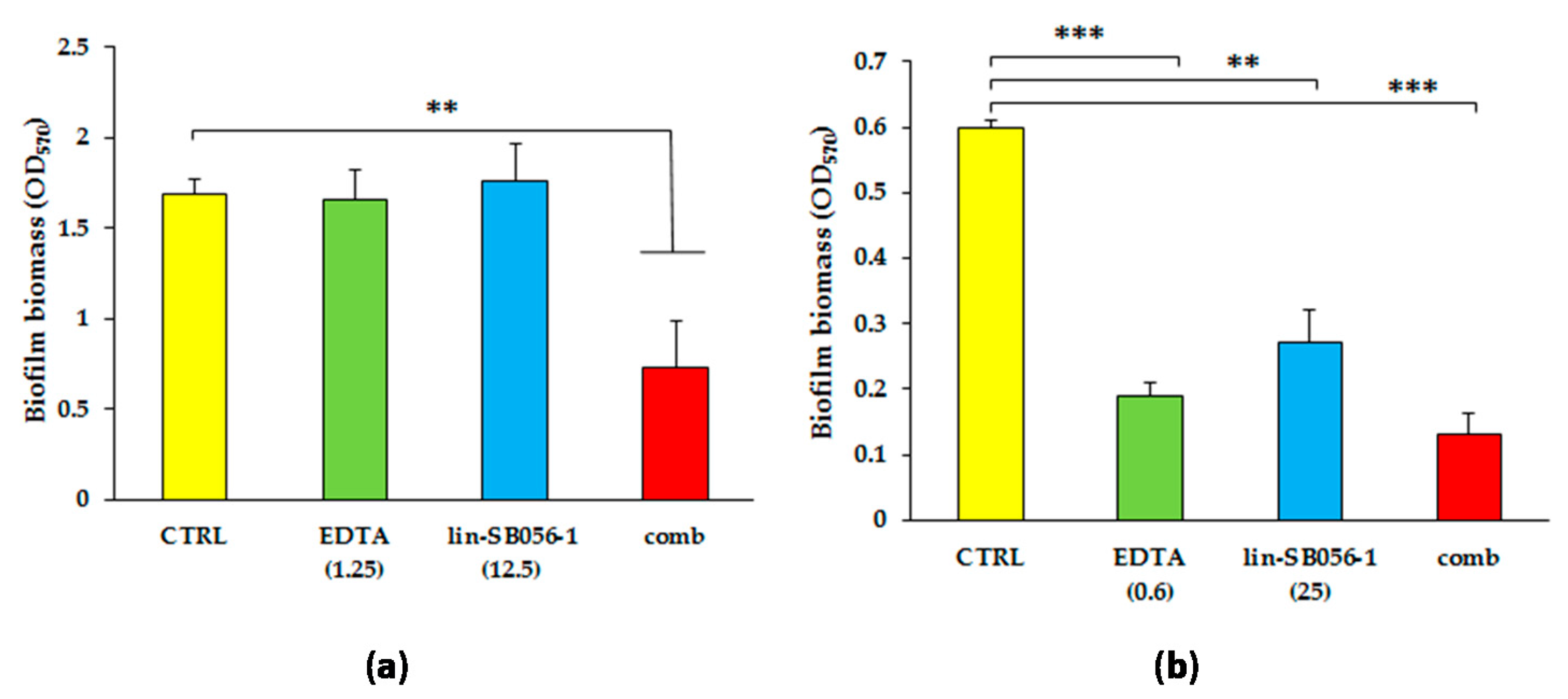
2.2. Synergistic Activity of Lin-SB056-1 in Combination with EDTA on Preformed Biofilms of Mucoid and Non-Mucoid Strains of P. aeruginosa
2.3. Bactericidal Effect of Lin-SB056-1 in Combination with EDTA against Planktonic P. aeruginosa in Artificial Sputum Medium (ASM)
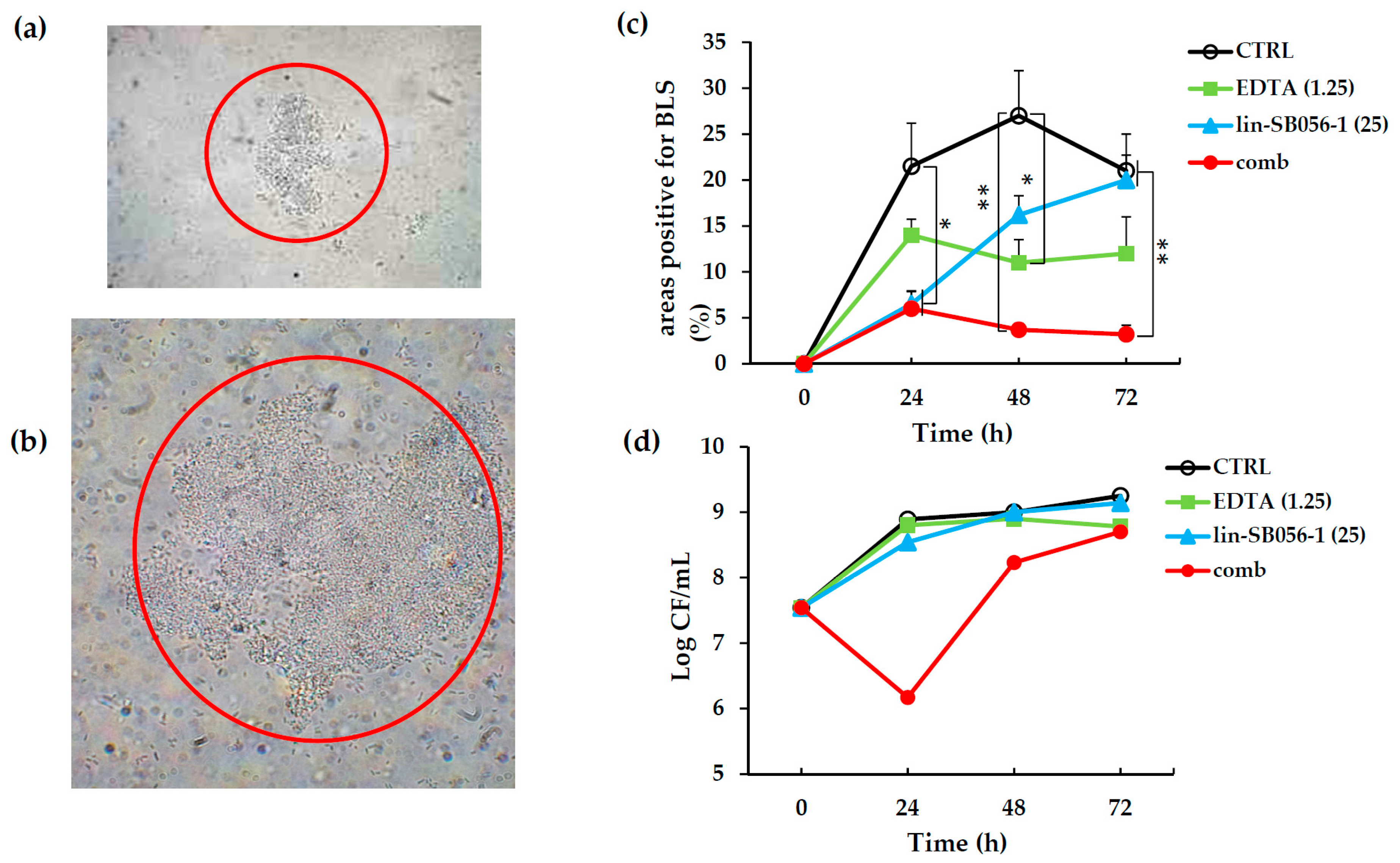
2.4. Activity of Lin-SB056-1 in Combination with EDTA in Preventing the Formation of Biofilm-Like Structures (BLSs) of P. aeruginosa ATCC 27853 in ASM
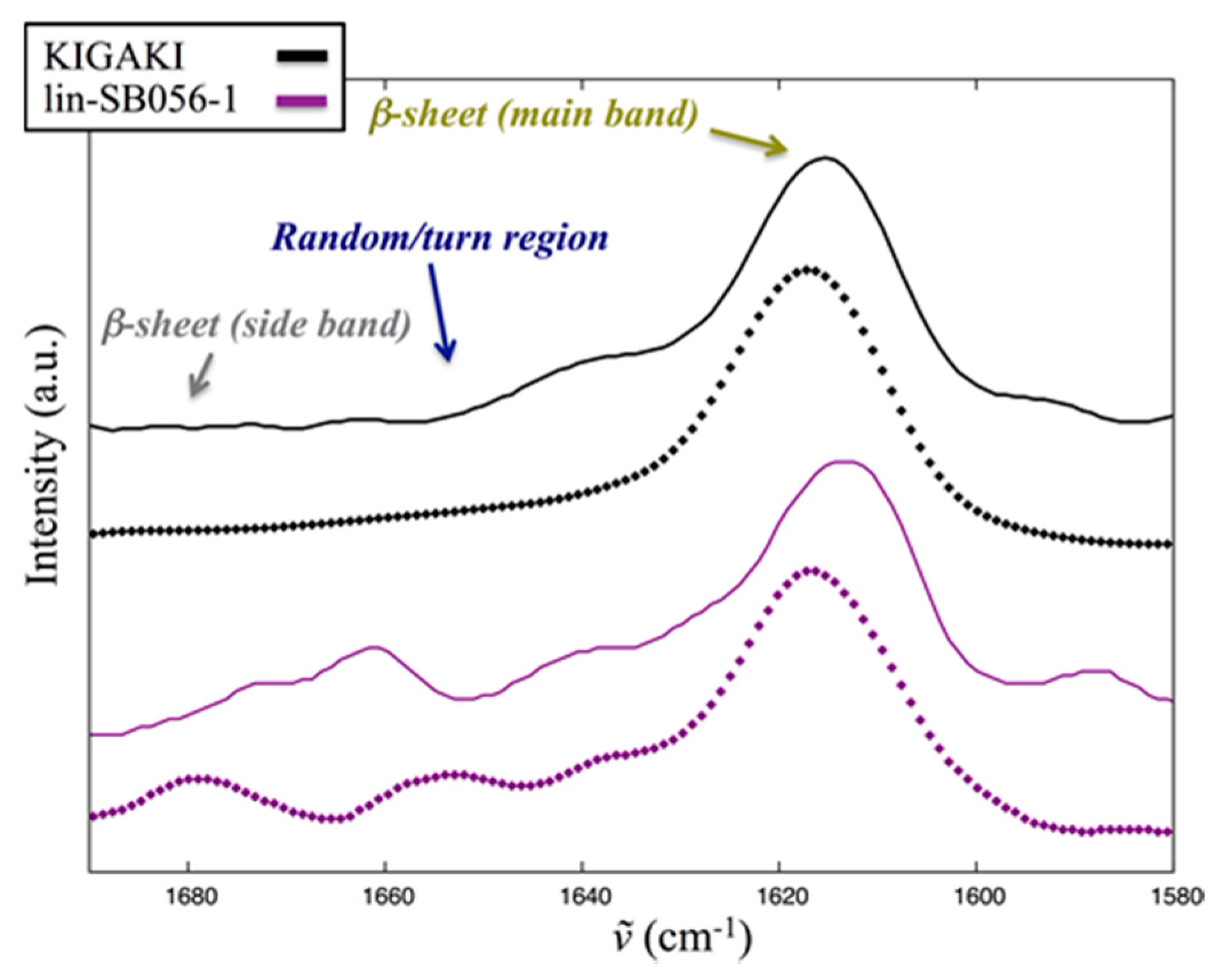
2.5. Interaction of Lin-SB056-1 with Lipid Vesicles in the Presence and Absence of EDTA, as Observed through Infrared Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Pseudomonas aeruginosa Strains and Culture Conditions
4.2. Peptide and EDTA Solutions
4.3. Infrared Spectroscopy
4.4. Bactericidal Activity in Sodium-Phosphate Buffer (SPB)
4.5. Biofilm Treatment Assays
4.6. Bactericidal Assays in ASM
4.7 Static Culture System for the Development of Biofilm-Like Structures (BLSs) in ASM
4.8. Visualization and Quantification of BLSs
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial peptide |
| ANOVA | Analysis of variance |
| ASM | Artificial sputum medium |
| BLS | Biofilm-like structure |
| CF | Cystic fibrosis |
| CFU | Colony-forming units |
| EDTA | Ethylenediaminetetraacetic acid |
| FH | Fully hydrated |
| LB | Luria bertani broth |
| LPS | Lipopolysaccharide |
| LUVs | Large unilamellar vesicles |
| MBC | Minimum bactericidal concentration |
| MDR | Multi-drug resistant |
| PB | Phosphate buffer |
| PBS | Phosphate buffered saline |
| POPC | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine |
| POPG | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) |
| RF | Rehydrated film |
| SPB | Sodium phosphate buffer |
| TSA | Tryptone soya agar |
| TSB | Tryptone soya broth |
References
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Mastoridis, P. Clinical implications of Pseudomonas aeruginosa location in the lungs of patients with cystic fibrosis. J. Clin. Pharm. Ther. 2017, 42, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.; O’Brien, S.; Brockhurst, M.A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Haley, C.L.; Colmer-Hamood, J.A.; Hamood, A.N. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiol. 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Aye, L.M.; Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007, 189, 8079–8087. [Google Scholar] [CrossRef] [PubMed]
- Sriramulu, D.D.; Lünsdorf, H.; Lam, J.S.; Römling, U. Microcolony formation: A novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J. Med. Microbiol. 2005, 54, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Langan, K.M.; Kotsimbos, T.; Peleg, A.Y. Managing Pseudomonas aeruginosa respiratory infections in cystic fibrosis. Curr. Opin. Infect. Dis. 2015, 28, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M. Antimicrobial peptides under clinical trials. Curr. Top. Med. Chem. 2017, 17, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K. Membrane-active antimicrobial peptides as template structures for novel antibiotic agents. Curr. Top. Med. Chem. 2017, 17, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Maccari, G.; Maisetta, G.; Batoni, G. BaAMPs: The database of biofilm-active antimicrobial peptides. Biofouling 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Casu, M.; Giuliani, A.; Luca, V.; Maisetta, G.; Mangoni, M.L.; Manzo, G.; Pintus, M.; Pirri, G.; Rinaldi, A.C.; et al. Rational modification of a dendrimeric peptide with antimicrobial activity: Consequences on membrane-binding and biological properties. Amino Acids 2016, 48, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Scorciapino, M.A.; Wadhwani, P.; Bürck, J.; Montaldo, N.P.; Pintus, M.; Sanna, R.; Casu, M.; Giuliani, A.; Pirri, G.; et al. Enhanced amphiphilic profile of a short β-stranded peptide improves its antimicrobial activity. PLoS ONE 2015, 10, e0116379. [Google Scholar] [CrossRef] [PubMed]
- Klinger-Strobel, M.; Lautenschläger, C.; Fischer, D.; Mainz, J.G.; Bruns, T.; Tuchscherr, L.; Pletz, M.W.; Makarewicz, O. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis-where do we stand? Expert Opin. Drug Deliv. 2015, 12, 1351–1374. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Hodges, N.A.; Marriott, C. Use of slime dispersants to promote antibiotic penetration through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1991, 35, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, S.; Percival, S.L. EDTA: An antimicrobial and antibiofilm agent for use in wound care. Adv. Wound Care (New Rochelle) 2015, 4, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, Y.; Lu, Q.; Li, F.; Yu, J.; Wang, Z.; He, Y.; Song, C. In vitro and in vivo activity of EDTA and antibacterial agents against the biofilm of mucoid Pseudomonas aeruginosa. Infection 2017, 45, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Leflon-Guibout, V.; Ghigo, J.M.; Beloin, C. In vitro activity of gentamicin, vancomycin or amikacin combined with EDTA or l-arginine as lock therapy against a wide spectrum of biofilm-forming clinical strains isolated from catheter-related infections. J. Antimicrob. Chemother. 2015, 70, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Beloin, C.; Renard, S.; Ghigo, J.M.; Lebeaux, D. Novel approaches to combat bacterial biofilms. Curr. Opin. Pharmacol. 2014, 18, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, L.; Maisetta, G.; Maccari, G.; Esin, S.; Batoni, G. Analogs of the frog-skin antimicrobial peptide temporin 1Tb exhibit a wider spectrum of activity and a stronger antibiofilm potential as compared to the parental peptide. Front. Chem. 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Haris, P.I.; Chapman, D. The conformational analysis of peptides using fourier transform IR spectroscopy. Biopolymers 1995, 37, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, H.; Kitagawa, T. FT-IR approaches on amyloid fibril structure. Biochim. Biophys. Acta 2005, 1753, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef] [PubMed]
- Blazyk, J.; Wiegand, R.; Klein, J.; Hammer, J.; Epand, R.M.; Epand, R.F.; Maloy, W.L.; Kari, U.P. A novel linear amphipathic β-sheet cationic antimicrobial peptide with enhanced selectivity for bacterial lipids. J. Biol. Chem. 2001, 276, 27899–27906. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Goormaghtigh, E.; Ruysschaert, J.M. Attenuated total reflection IR spectroscopy as a tool to investigate the orientation and tertiary structure changes in fusion proteins. Biochim. Biophys. Acta 2003, 1614, 97–103. [Google Scholar] [CrossRef]
- Menikh, A.; Saleh, M.T.; Gariépy, J.; Boggs, J.M. Orientation in lipid bilayers of a synthetic peptide representing the C-terminus of the A1 domain of Shiga toxin. A polarized ATR-FTIR study. Biochemistry 1997, 36, 15865–15872. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Rutter, W.C.; Burgess, D.R.; Burgess, D.S. Increasing incidence of multidrug resistance among cystic fibrosis respiratory bacterial isolates. Microb. Drug Resist. 2017, 23, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sherrard, L.J.; Tunney, M.M.; Elborn, J.S. Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. Lancet 2014, 384, 703–713. [Google Scholar] [CrossRef]
- Waters, V.; Smyth, A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015, 14, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Crocetta, V.; Scocchi, M.; Pomponio, S.; di Vincenzo, V.; Mardirossian, M.; Gherardi, G.; Fiscarelli, E.; Dicuonzo, G.; Gennaro, R.; et al. Potential novel therapeutic strategies in cystic fibrosis: Antimicrobial and anti-biofilm activity of natural and designed α-helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; di Grazia, A.; Segev-Zarko, L.A.; Scali, S.; Ferrera, L.; Galietta, L.; Pini, A.; Shai, Y.; Di, Y.P.; Mangoni, M.L. Esculentin-1a-derived peptides promote clearance of Pseudomonas aeruginosa internalized in bronchial cells of cystic fibrosis patients and lung cell migration: Biochemical properties and a plausible mode of action. Antimicrob. Agents Chemother. 2016, 60, 7252–7262. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.E.; Dubois, A.V.; Ingram, R.J.; Weldon, S.; Taggart, C.C.; Elborn, J.S.; Tunney, M.M. Activity of innate antimicrobial peptides and ivacaftor against clinical cystic fibrosis respiratory pathogens. Int. J. Antimicrob. Agents. 2017, 50, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Di Luca, M.; Esin, S.; Florio, W.; Brancatisano, F.L.; Bottai, D.; Campa, M.; Batoni, G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human β defensin 3. Peptides 2008, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Cohen, H.; Casciaro, B.; Luca, V.; Pini, A.; Di, Y.P.; Shai, Y.; Mangoni, M.L. d-Amino acids incorporation in the frog skin-derived peptide esculentin-1a(1–21)NH2 is beneficial for its multiple functions. Amino Acids 2015, 47, 2505–2519. [Google Scholar] [CrossRef] [PubMed]
- Scorciapino, M.A.; Pirri, G.; Vargiu, A.V.; Ruggerone, P.; Giuliani, A.; Casu, M.; Buerck, J.; Wadhwani, P.; Ulrich, A.S.; Rinaldi, A.C. A novel dendrimeric peptide with antimicrobial properties: Structure-function analysis of SB056. Biophys. J. 2012, 102, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.; Waring, A.J.; Hong, M. Dynamic structure of disulfide-removed linear analogs of Tachyplesin-I in the lipid bilayer from solid-state NMR. Biochemistry 2008, 47, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; The sticky side of biofilm formation. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Goltermann, L.; Tolker-Nielsen, T. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob. Agents Chemother. 2017, 61, e02696-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, Z.; Hentzer, M.; Andersen, J.B.; Molin, S.; Givskov, M.; Høiby, N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 2004, 53, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Vandeputte, O.M.; Pottier, L.; Huet, J.; Rabemanantsoa, C.; Kiendrebeogo, M.; Andriantsimahavandy, A.; Rasamindrakotroka, A.; Stévigny, C.; Duez, P.; et al. Pseudomonas aeruginosa biofilm formation and persistence, along with the production of quorum sensing-dependent virulence factors, are disrupted by a triterpenoid coumarate ester isolated from Dalbergia trichocarpa, a tropical legume. PLoS ONE 2015, 10, e0132791. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Chen, L.; Liu, H.; Zou, Y.; Luo, Z.; Koronfel, A.; Wu, Y. The effects of d-Tyrosine combined with amikacin on the biofilms of Pseudomonas aeruginosa. Microb. Pathog. 2015, 86, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K.P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Born, T.; Kontoghiorghe, C.N.; Spyrou, A.; Kolnagou, A.; Kontoghiorghes, G.J. EDTA chelation reappraisal following new clinical trials and regular use in millions of patients: Review of preliminary findings and risk/benefit assessment. Toxicol. Mech. Methods 2013, 23, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.; Bahna, P.; Hanna, H.; Stephens, L.C.; Raad, I. EDTA as an adjunct antifungal agent for invasive pulmonary aspergillosis in a rodent model. Antimicrob. Agents Chemother. 2006, 50, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, S.; Patrauchan, M.A.; Berglund, D.; Nivens, D.E.; Franklin, M.J. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 2005, 187, 4327–4337. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.; Fothergill, J.L.; Wright, E.A.; James, C.E.; Mowat, E.; Winstanley, C. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J. Vis. Exp. 2012, 64, e3857. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Naughton, S.; Turnbull, L.; Tingpej, P.; Rose, B.; Arthur, J.; Hu, H.; Harmer, C.; Harbour, C.; Hassett, D.J.; et al. Gene expression of Pseudomonas aeruginosa in a mucin-containing synthetic growth medium mimicking cystic fibrosis lung sputum. J. Med. Microbiol. 2010, 59, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjövall, H.; Hansson, G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp Med. 2012, 209, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.A.; Nolan, W.G. Elemental analysis of vitamin-free casamino acids. Appl. Microbiol. 1972, 24, 290–291. [Google Scholar] [PubMed]
- Alhede, M.; Kragh, K.N.; Qvortrup, K.; Allesen-Holm, M.; van Gennip, M.; Christensen, L.D.; Jensen, P.Ø.; Nielsen, A.K.; Parsek, M.; Wozniak, D.; et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS ONE 2011, 6, e27943. [Google Scholar] [CrossRef] [PubMed]
- Kragh, K.N.; Hutchison, J.B.; Melaugh, G.; Rodesney, C.; Roberts, A.E.; Irie, Y.; Jensen, P.Ø.; Diggle, S.P.; Allen, R.J.; Gordon, V.; et al. Role of multicellular aggregates in biofilm formation. MBio 2016, 7, e00237. [Google Scholar] [CrossRef] [PubMed]
- O’May, C.Y.; Sanderson, K.; Roddam, L.F.; Kirov, S.M.; Reid, D.W. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J. Med. Microbiol. 2009, 58, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Grassi, L.; di Luca, M.; Bombardelli, S.; Medici, C.; Brancatisano, F.L.; Esin, S.; Batoni, G. Anti-biofilm properties of the antimicrobial peptide temporin 1Tb and its ability, in combination with EDTA, to eradicate Staphylococcus epidermidis biofilms on silicone catheters. Biofouling 2016, 32, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Serra, I.; Pira, A.; Pintus, M.; Ceccarelli, M.; Casu, M.; Rinaldi, A.C.; Scorciapino, M.A. The singular behavior of a β-type semi-synthetic two branches polypeptide. Three-dimensional structure and mode of action. Phys. Chem. Chem. Phys. 2016, 18, 30998–31011. [Google Scholar] [CrossRef] [PubMed]




| Strain | Phenotype | Resistance Profile 1 | MBC 2 |
|---|---|---|---|
| ATCC 27853 | Non-mucoid | None | 3.12 |
| PaNM01 | Non-mucoid | FOS | 3.12 |
| PaNM02 | Non-mucoid | FOS | 3.12 |
| PaM01 | Mucoid | AMC-AZT-CIP-CTX-ERT-LVX-SAM-TIG-TOB | 1.56 |
| PaM02 | Mucoid | AMK-CIP-FEP-FOS-GEN-LVX-TOB | 1.56 |
| PaM03 | Mucoid | FOS-LVX-TOB | 3.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisetta, G.; Grassi, L.; Esin, S.; Serra, I.; Scorciapino, M.A.; Rinaldi, A.C.; Batoni, G. The Semi-Synthetic Peptide Lin-SB056-1 in Combination with EDTA Exerts Strong Antimicrobial and Antibiofilm Activity against Pseudomonas aeruginosa in Conditions Mimicking Cystic Fibrosis Sputum. Int. J. Mol. Sci. 2017, 18, 1994. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091994
Maisetta G, Grassi L, Esin S, Serra I, Scorciapino MA, Rinaldi AC, Batoni G. The Semi-Synthetic Peptide Lin-SB056-1 in Combination with EDTA Exerts Strong Antimicrobial and Antibiofilm Activity against Pseudomonas aeruginosa in Conditions Mimicking Cystic Fibrosis Sputum. International Journal of Molecular Sciences. 2017; 18(9):1994. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091994
Chicago/Turabian StyleMaisetta, Giuseppantonio, Lucia Grassi, Semih Esin, Ilaria Serra, Mariano A. Scorciapino, Andrea C. Rinaldi, and Giovanna Batoni. 2017. "The Semi-Synthetic Peptide Lin-SB056-1 in Combination with EDTA Exerts Strong Antimicrobial and Antibiofilm Activity against Pseudomonas aeruginosa in Conditions Mimicking Cystic Fibrosis Sputum" International Journal of Molecular Sciences 18, no. 9: 1994. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091994