Mouse Monoclonal Antibodies Generated from Full Length Human Cereblon: Detection of Cereblon Protein in Patients with Multiple Myeloma
Abstract
:1. Introduction
2. Results
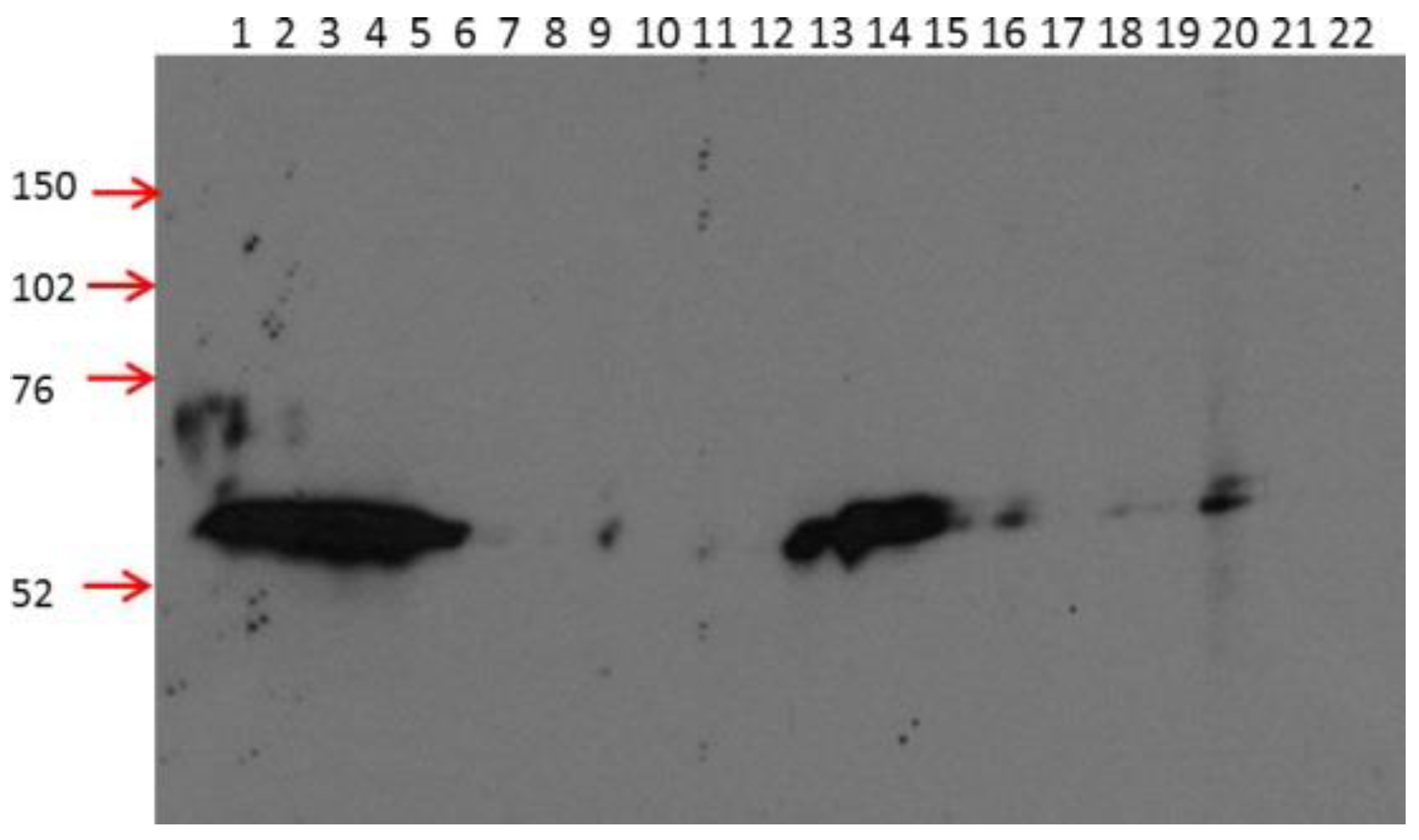
2.1. 2B11G10, 2F11G5 and 4B9D3 are Highly Specific mAbs for Detecting Human CRBN in Western Blot Analysis
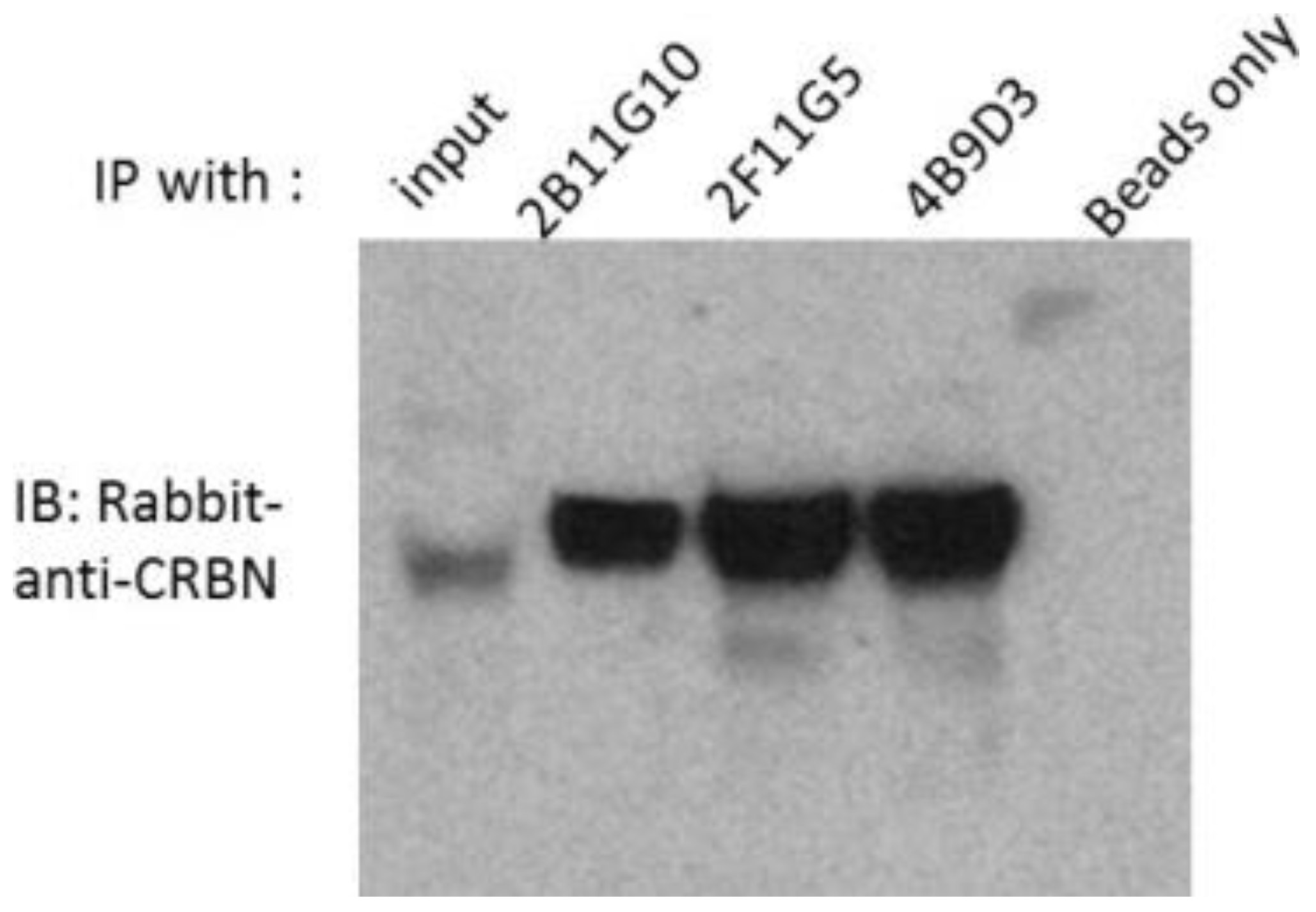
2.2. 2B11G10, 2F11G5 and 4B9D3 Can Immunoprecipitate Human CRBN from Multiple Myeloma Cell Lysates
2.3. Determination of Epitopes for 2B11G10, 2F11G5 and 4B9D3
2.4. Immunohistochemistry (IHC) Staining of Multiple Myeloma Cell Lines with mAb 2F11G5
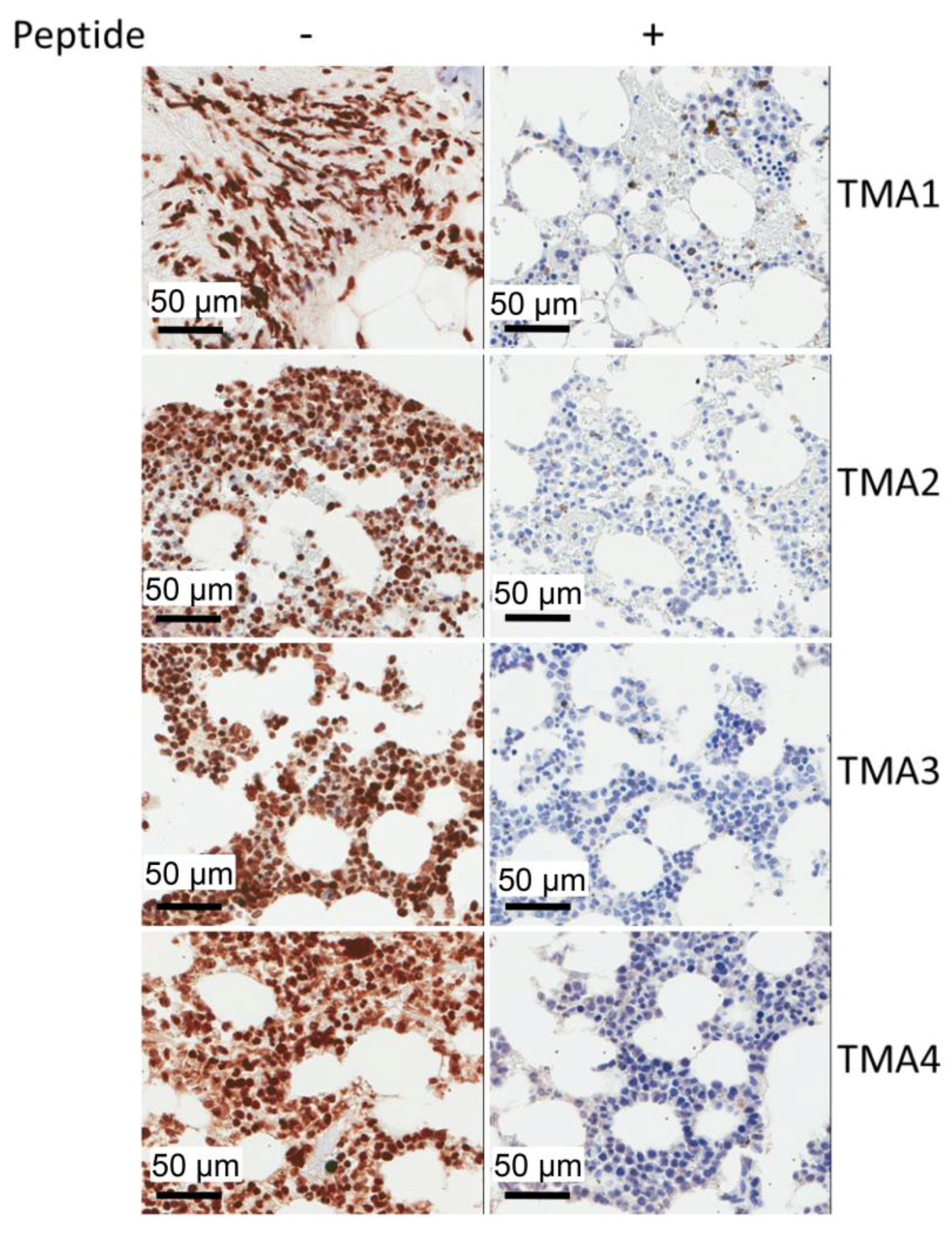
2.5. IHC Staining of Multiple Myeloma Patients’ Specimens with mAb 2F11G5
3. Discussion
4. Materials and Methods
4.1. Multiple Myeloma Cell Lines and Cell Culture
4.2. Generation of Monoclonal Antibodies (mAbs) against Human CRBN
4.3. Generation of Recombinant CRBN Proteins
4.4. Immunoblotting and Immunoprecipitation
4.5. IHC Staining
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhu, Y.; Shi, C.; Stewart, A.K. Mechanism of immunomodulatory drugs’ action in the treatment of multiple myeloma. Acta Biochim. Biophys. Sin. 2014, 46, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K. Novel therapies for relapsed myeloma. Hematol. Am. Soc. Hematol. Educ. Program 2009. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Angers, S.; Li, T.; Yi, X.; MacCoss, M.J.; Moon, R.T.; Zheng, N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 2006, 443, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.B.; Stewart, A.K. What is the functional role of the thalidomide binding protein cereblon? Int. J. Biochem. Mol. Biol. 2011, 2, 287–294. [Google Scholar] [PubMed]
- Jo, S.; Lee, K.H.; Song, S.; Jung, Y.K.; Park, C.S. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. J. Neurochem. 2005, 94, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.J.; Tal, A.L.; Sun, X.; Hauck, S.C.; Hao, J.; Kosofosky, B.E.; Rajadhyaksha, A.M. Temporal and spatial mouse brain expression of cereblon, an ionic channel regulator involved in human intelligence. J. Neurogenet. 2010, 24, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Enz, R. Cereblon is expressed in the retina and binds to voltage-gated chloride channels. FEBS Lett. 2009, 583, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Jo, S.; Kim, H.; Lee, J.; Park, C.S. Functional modulation of AMP-activated protein kinase by cereblon. Biochim. Biophys. Acta 2011, 1813, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, J.; Park, C.S. Cereblon inhibits proteasome activity by binding to the 20S core proteasome subunit beta type 4. Biochem. Biophys. Res. Commun. 2012, 427, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G., Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Braggio, E.; Shi, C.X.; Kortuem, K.M.; Bruins, L.A.; Schmidt, J.E.; Chang, X.B.; Langlais, P.; Luo, M.; Jedlowski, P.; et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 2014, 124, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Kronke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.K.; Kang, J.; Havens, C.G.; Conklin, T.; Ning, Y.; Wu, L.; Ito, T.; Ando, H.; Waldman, M.F.; Thakurta, A.; et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br. J. Haematol. 2014, 164, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.S.; Bohm, K.; Lydeard, J.R.; Yang, H.; Stadler, M.B.; Cavadini, S.; Nagel, J.; Serluca, F.; Acker, V.; Lingaraju, G.M.; et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hou, Y.X.; Langlais, P.; Erickson, P.; Zhu, J.; Shi, C.X.; Luo, M.; Zhu, Y.; Xu, Y.; Mandarino, L.J.; et al. Expression of the cereblon binding protein argonaute 2 plays an important role for multiple myeloma cell growth and survival. BMC Cancer 2016, 16, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Braggio, E.; Shi, C.X.; Bruins, L.A.; Schmidt, J.E.; Van Wier, S.; Chang, X.B.; Bjorklund, C.C.; Fonseca, R.; Bergsagel, P.L.; et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011, 118, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Broyl, A.; Kuiper, R.; van Duin, M.; van der Holt, B.; el Jarari, L.; Bertsch, U.; Zweegman, S.; Buijs, A.; Hose, D.; Lokhorst, H.M.; et al. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood 2013, 121, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Heintel, D.; Rocci, A.; Ludwig, H.; Bolomsky, A.; Caltagirone, S.; Schreder, M.; Pfeifer, S.; Gisslinger, H.; Zojer, N.; Jager, U.; et al. High expression of cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone. Br. J. Haematol. 2013, 161, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.R.; Kortuem, K.M.; Zhu, Y.X.; Braggio, E.; Shi, C.X.; Bruins, L.A.; Schmidt, J.E.; Ahmann, G.; Kumar, S.; Rajkumar, S.V.; et al. The clinical significance of cereblon expression in multiple myeloma. Leuk. Res. 2014, 38, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jonasova, A.; Bokorova, R.; Polak, J.; Vostry, M.; Kostecka, A.; Hajkova, H.; Neuwirtova, R.; Siskova, M.; Sponerova, D.; Cermak, J.; et al. High level of full-length cereblon mRNA in lower risk myelodysplastic syndrome with isolated 5q deletion is implicated in the efficacy of lenalidomide. Eur. J. Haematol. 2015, 95, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Lin, C.W.; Lin, H.H.; Yao, M.; Tang, J.L.; Wu, S.J.; Chen, Y.C.; Lu, H.Y.; Hou, H.A.; Chen, C.Y.; et al. Expression of cereblon protein assessed by immunohistochemicalstaining in myeloma cells is associated with superior response of thalidomide- and lenalidomide-based treatment, but not bortezomib-based treatment, in patients with multiple myeloma. Ann. Hematol. 2014, 93, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.K.; Mendy, D.; Waldman, M.; Chen, G.; Rychak, E.; Miller, K.; Gaidarova, S.; Ren, Y.; Wang, M.; Breider, M.; et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br. J. Haematol. 2014, 164, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, N.; Wakabayashi, S.; Matsumoto, K.; Yamada, H.; Asahi, T. Cereblon is recruited to aggresome and shows cytoprotective effect against ubiquitin-proteasome system dysfunction. Biochem. Biophys. Res. Commun. 2015, 464, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005, 352, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, M.; Couto, S.; Hansel, D.E.; Miller, K.; Lopez-Girona, A.; Bjorklund, C.C.; Gandhi, A.K.; Thakurta, A.; Chopra, R.; et al. A Dual Color Immunohistochemistry Assay for Measurement of Cereblon in Multiple Myeloma Patient Samples. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Asahi, T.; Sawamura, N. Nuclear cereblon modulates transcriptional activity of Ikaros and regulates its downstream target, enkephalin, in human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2016, 477, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nakamura, C.; Asahi, T.; Sawamura, N. Mitochondrial cereblon functions as a Lon-type protease. Sci. Rep. 2016, 6, 29986. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cui, L.; Riordan, J.R.; Chang, X.B. Allosteric interactions between the two non-equivalent nucleotide binding domains of multidrug resistance protein MRP1. J. Biol. Chem. 2000, 275, 20280–20287. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhou, P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol. Cell 2007, 26, 775–780. [Google Scholar] [CrossRef] [PubMed]



| Number | Name | Oligonucleotide Sequence (from 5′ to 3′) |
|---|---|---|
| 1 | CRBN.42.4.RV | GCGGCCGCTCACAGCTGACATCCAAAAAGGATGTTTTCTCGCAAGCAAAGTATTACTTTG |
| 2 | CRBN.N-half.FW | GAAATACCAGAAGAGAAAGTTTCGAGAAAACATCCTTTTTGG |
| 3 | CRBN.N-half.RV | CCAAAAAGGATGTTTTCTCGAAACTTTCTCTTCTGGTATTTC |
| 4 | CRBN.C-half.FW | GACGACGACGACAAGGCCATGCATTGTGCAAATCTAACTTCA |
| 5 | CRBN.C-half.RV | TGAAGTTAGATTTGCACAATGCATGGCCTTGTCGTCGTCGTC |
| 6 | CRBN.110/330.FW | CCTCAAGAAGTCAGTATGGTGGAAATAACAACCAAAAATG |
| 7 | CRBN.110/330.RV | CATTTTTGGTTGTTATTTCCACCATACTGACTTCTTGAGG |
| 8 | d20/233.FW | GGCAACCACCTGCCGCTCCATTGTGCAAATCTAACTTC |
| 9 | d20/233.RV | GAAGTTAGATTTGCACAATGGAGCGGCAGGTGGTTGCC |
| 10 | d40/233.FW | GACCAGGATAGTAAAGAACATTGTGCAAATCTAACTTC |
| 11 | d40/233.RV | GAAGTTAGATTTGCACAATGTTCTTTACTATCCTGGTC |
| 12 | d60/233.FW | CATCACATACATACCTACATTGTGCAAATCTAACTTC |
| 13 | d60/233.RV | GAAGTTAGATTTGCACAATGTAGGTATGTATGTGATG |
| 14 | d70/233.FW | GAAGAATTTCATGGCAGGCATTGTGCAAATCTAACTTC |
| 15 | d70/233.RV | GAAGTTAGATTTGCACAATGCCTGCCATGAAATTCTTC |
| 16 | d80/233.FW | GACGACAGCTGTCAGGTGCATTGTGCAAATCTAACTTC |
| 17 | d80/233.RV | GAAGTTAGATTTGCACAATGCACCTGACAGCTGTCGTC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Xu, Q.; Hou, Y.; Li, C.; Xu, Y.; Stewart, A.K. Mouse Monoclonal Antibodies Generated from Full Length Human Cereblon: Detection of Cereblon Protein in Patients with Multiple Myeloma. Int. J. Mol. Sci. 2017, 18, 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091999
Chang X, Xu Q, Hou Y, Li C, Xu Y, Stewart AK. Mouse Monoclonal Antibodies Generated from Full Length Human Cereblon: Detection of Cereblon Protein in Patients with Multiple Myeloma. International Journal of Molecular Sciences. 2017; 18(9):1999. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091999
Chicago/Turabian StyleChang, Xiubao, Qinqin Xu, Yuexian Hou, Cynthia Li, Ye Xu, and A. Keith Stewart. 2017. "Mouse Monoclonal Antibodies Generated from Full Length Human Cereblon: Detection of Cereblon Protein in Patients with Multiple Myeloma" International Journal of Molecular Sciences 18, no. 9: 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091999




