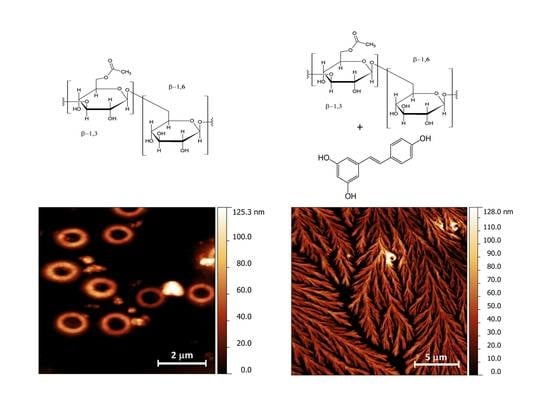
Behind Resveratrol Stabilization by Carboxymethylated (1,3/1,6)-β-d-Glucan: Does the Polyphenol Play a Role in Polymer Structural Organization?
Abstract
:1. Introduction
2. Results and Discussion
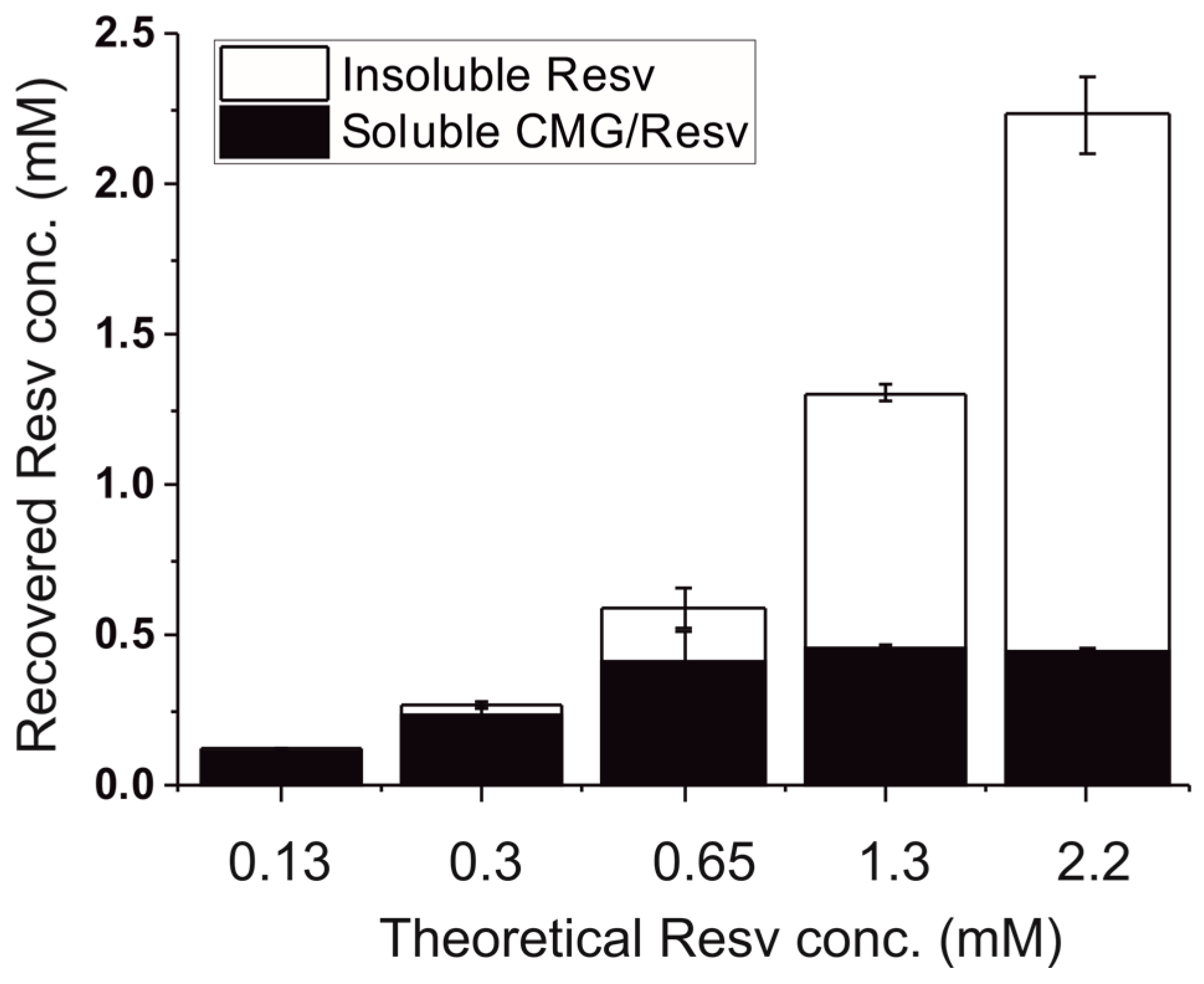
2.1. Solubility of Resveratrol in CM-Glucan Aqueous Solution
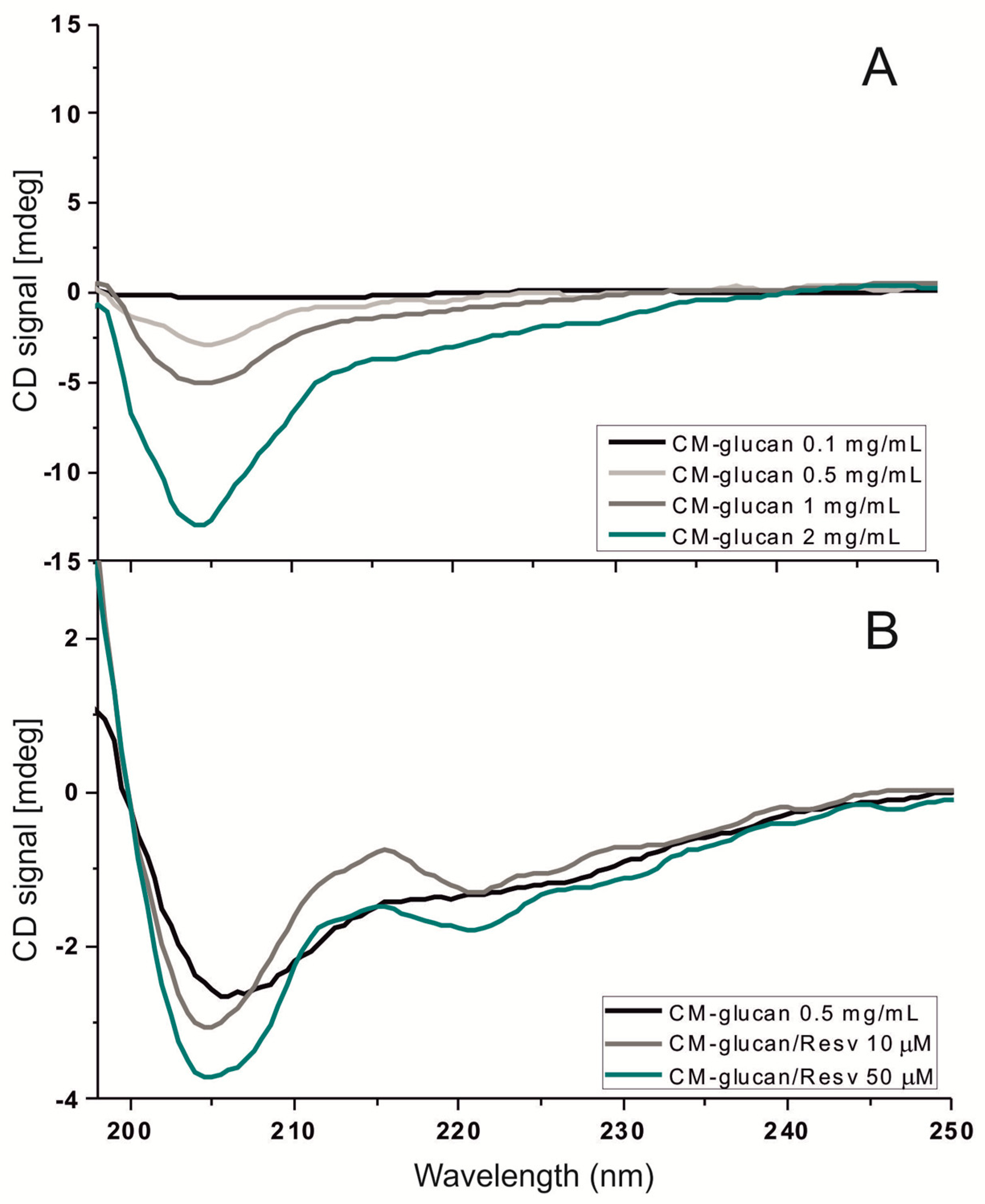
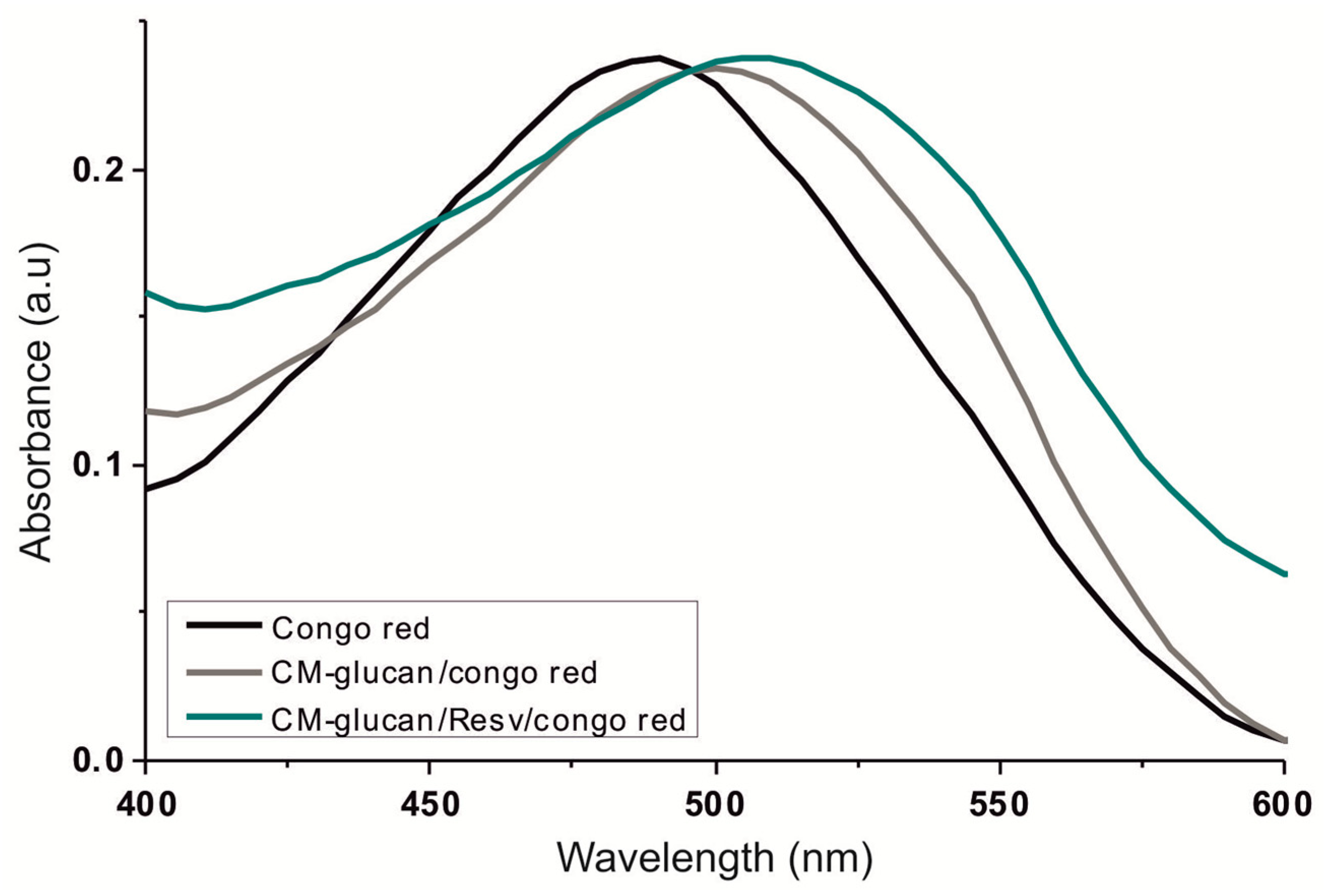
2.2. Spectroscopic Studies of CM-Glucan/Resveratrol Complex
2.3. Thermal Transitions of CM-Glucan
2.4. Infrared Analyses
2.5. Fluorescence and Binding Studies
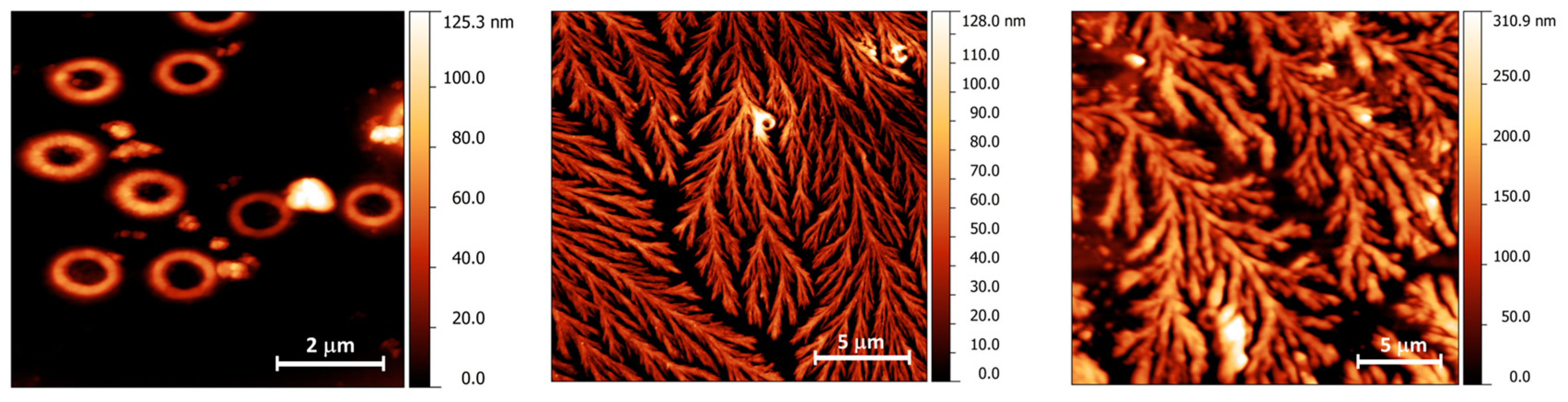
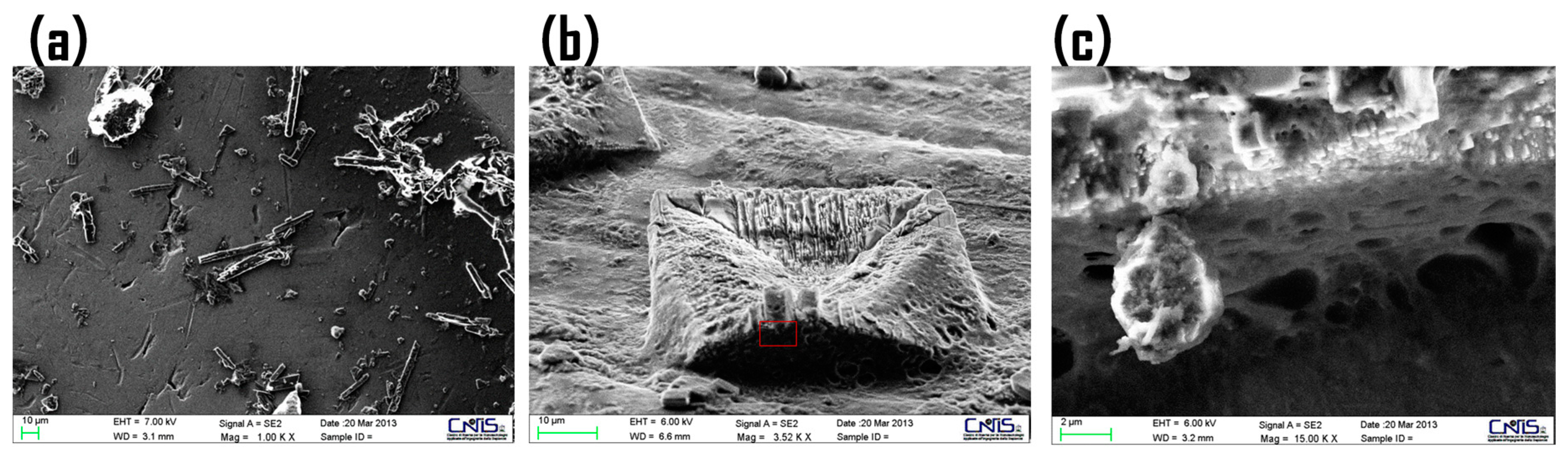
2.6. Morphological Studies of CM-Glucan/Resveratrol Complex
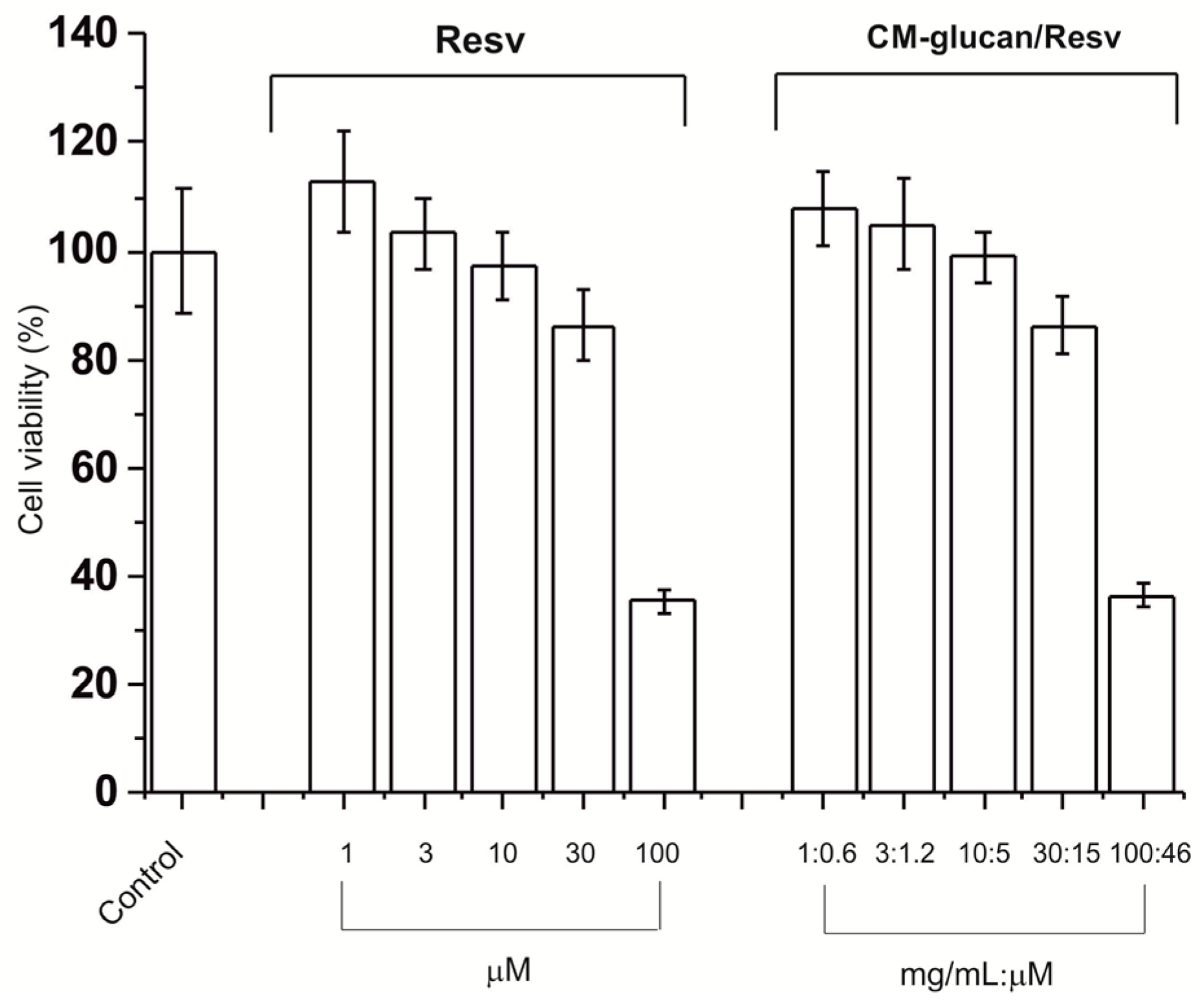
2.7. Cell Viability of Resveratrol/CM-Glucan Association
3. Materials and Methods
3.1. Solubility Studies
3.2. Congo Red Binding Assay
3.3. Circular Dichroism (CD)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Fourier Transform Infrared Spectroscopy (FT-IR)
3.6. Fluorescence binding Study
3.7. Atomic Force Microscopy (AFM)
3.8. Scanning Electron Microscopy (SEM) and Elemental Analyses
3.9. MTT Cell Viability Assay
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| SEM | Scanning Electron Microscopy |
| DSC | Differential Scanning Calorimetry |
| CD | Circular Dichroism |
References
- Pizzoferrato, L.; Manzi, P.; Bertocchi, F.; Fanelli, C.; Rotilio, G.; Paci, M. Solid-state C-13 CP MAS NMR spectroscopy of mushrooms gives directly the ratio between proteins and polysaccharides. J. Agric. Food Chem. 2000, 48, 5484–5488. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Pizzoferrato, L. β-glucans in edible mushrooms. Food Chem. 2000, 68, 315–318. [Google Scholar] [CrossRef]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.M.; Burton, R.A.; Topping, D.L.; Liao, M.L.; Bacic, A.; Fincher, G.B. Variability in fine structures of noncellulosic cell wall polysaccharides from cereal grains: Potential importance in human health and nutrition. Cereal Chem. 2010, 87, 272–282. [Google Scholar] [CrossRef]
- Sutherland, I.W. Microbial polysaccharides from gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Wiater, A.; Paduch, R.; Pleszczynska, M.; Prochniak, K.; Choma, A.; Kandefer-Szerszen, M.; Szczodrak, J. α-(1→3)-d-glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol. Lett. 2011, 33, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kiemle, S.N.; Zhang, X.; Esker, A.R.; Toriz, G.; Gatenholm, P.; Cosgrove, D.J. Role of (1,3)(1,4)-β-glucan in cell walls: Interaction with cellulose. Biomacromolecules 2014, 15, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Lehtovaara, B.C.; Gu, F.X. Pharmacological, structural, and drug delivery properties and applications of 1,3-β-glucans. J. Agric. Food Chem. 2011, 59, 6813–6828. [Google Scholar] [CrossRef] [PubMed]
- Magnani, M.; Castro-Gomez, R.H.; Aoki, M.N.; Gregorio, E.P.; Libos, F.; Morimoto, H.K.; Reiche, E.M.V.; Watanabe, M.A.E. Analysis of peripheral T cells and the CC chemokine receptor (CCR5) delta32 polymorphism in prostate cancer patients treated with carboxymethyl-glucan (CM-G). Nat. Prod. Res. 2012, 26, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Kanke, M.; Katayama, H.; Nakamura, M. Application of curdlan to controlled drug-delivery.2. In-vitro and in-vivo drug-release studies of theophylline-containing curdlan tablets. Biol. Pharm. Bull. 1995, 18, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, F.P.; Tang, H.B.; Bai, Y.G.; Li, R.F.; Li, X.M.; Liu, L.R.; Wang, Y.S.; Zhang, Q.Q. Self-assembled nanoparticles of cholesterol-conjugated carboxymethyl curdlan as a novel carrier of epirubicin. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Sandula, J.; Kogan, G.; Kacurakova, M.; Machova, E. Microbial (1→3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohyd. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Babincova, M.; Bacova, Z.; Machova, E.; Kogan, G. Antioxidant properties of carboxymethyl glucan: Comparative analysis. J. Med. Food 2002, 5, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Yu, Y.; Cheung, P.C. Enhancement of antitumor activities in sulfated and carboxymethylated polysaccharides of ganoderma lucidum. J. Agric. Food Chem. 2009, 57, 10565–10572. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Glucan-resveratrol-vitamin C combination offers protection against toxic agents. Toxins 2012, 4, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Combination of glucan, resveratrol and vitamin c demonstrates strong anti-tumor potential. Anticancer Res. 2012, 32, 81–87. [Google Scholar] [PubMed]
- Ross, G.D.; Vetvicka, V.; Yan, J.; Xia, Y.; Vetvickova, J. Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacology 1999, 42, 61–74. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Restignoli, R.; Boffi, A.; d’Erme, M.; Mosca, L. Improved stability of trans-resveratrol in aqueous solutions by carboxymethylated (1,3/1,6)-β-d-glucan. J. Agric. Food Chem. 2014, 62, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.R.; Rao, L.Q.; Yu, H.Z.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, L.R.; Jiang, Q.; Zhang, Q.Q. Self-aggregated nanoparticles of cholesterol-modified chitosan conjugate as a novel carrier of epirubicin. Eur. Polym. J. 2007, 43, 43–51. [Google Scholar] [CrossRef]
- Ogawa, K.; Dohmaru, T.; Yui, T. Dependence of complex-formation of (1→3)-β-d-glucan with congo red on temperature in alkaline-solutions. Biosci. Biotech. Bioch. 1994, 58, 1870–1872. [Google Scholar] [CrossRef]
- Qin, F.; Sletmoen, M.; Stokke, B.T.; Christensen, B.E. Higher order structures of a bioactive, water-soluble (1→3)-β-d-glucan derived from saccharomyces cerevisiae. Carbohyd. Polym. 2013, 92, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, H.P.; Tharanathan, R.N. Structural characteristics of a mixed linkage β-d-glucan from sorghum (sorghum bicolor). Carbohyd. Res. 1998, 308, 239–243. [Google Scholar] [CrossRef]
- Sakurai, K.; Uezu, K.; Numata, M.; Hasegawa, T.; Li, C.; Kaneko, K.; Shinkai, S. β-1,3-glucan polysaccharides as novel one-dimensional hosts for DNA/RNA, conjugated polymers and nanoparticles. Chem. Commun. 2005, 35, 4383–4398. [Google Scholar] [CrossRef] [PubMed]
- Bae, A.H.; Numata, M.; Hasegawa, T.; Li, C.; Kaneko, K.; Sakurai, K.; Shinkai, S. 1D arrangement of Au nanoparticles by the helical structure of schizophyllan: A unique encounter of a natural product with inorganic compounds. Angew. Chem. Int. Ed. 2005, 44, 2030–2033. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Shinkai, S. Phase separation in the mixture of schizophyllan and poly(ethylene oxide) in aqueous solution driven by a specific interaction between the glucose side chain and poly(ethylene oxide). Carbohydr. Res. 2000, 324, 136–140. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, L.; Ding, Y. Multiple conformation transitions of triple helical lentinan in dmso/water by microcalorimetry. J. Phys. Chem. B 2009, 113, 9915–9923. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Xu, X.J.; Zheng, H.; Li, J.L.; Deng, C.; Xu, Z.H.; Chen, J.H. Structural characterization, chain conformation, and morphology of a β-(1→3)-d-glucan isolated from the fruiting body of dictyophora indusiata. J. Agric. Food Chem. 2009, 57, 5918–5924. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; LeBoef, E.J.; Dai, S.; Gu, B.H. Fluorescence spectroscopic studies of natural organic matter fractions. Chemosphere 2003, 50, 639–647. [Google Scholar] [CrossRef]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochem. 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of β-lactoglobulin with resveratrol and its biological implications. Biomacromolecules 2008, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sletmoen, M.; Stokke, B.T. Review: Higher order structure of (1,3)-β-d-glucans and its influence on their biological activities and complexation abilities. Biopolym. 2008, 89, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J.; Erfle, J.D.; Teather, R.M. Use of complex-formation between congo red and polysaccharides in detection and assay of polysaccharide hydrolases. Method Enzymol. 1988, 160, 59–74. [Google Scholar]
- Guharay, J.; Sengupta, B.; Sengupta, P.K. Protein-flavonol interaction: Fluorescence spectroscopic study. Proteins 2001, 43, 75–81. [Google Scholar] [CrossRef]
- Futatsuyama, H.; Yui, T.; Ogawa, K. Viscometry of curdlan, a linear (1→3)-β-d-glucan, in dmso or alkaline solutions. Biosci. Biotech. Bioch. 1999, 63, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Stokke, B.T.; Elgsaeter, A.; Brant, D.A.; Kuge, T.; Kitamura, S. Macromolecular cyclization of (1→6)-branched-(1→3)-β-d-glucans observed after denaturation renaturation of the triple-helical structure. Biopolymer 1993, 33, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Chen, P.; Zhang, L.N.; Ashida, H. Chain structures of glucans from lentinus edodes and their effects on no production from raw 264.7 macrophages. Carbohyd. Polym. 2012, 87, 1855–1862. [Google Scholar] [CrossRef]
- Sletmoen, M.; Christensen, B.E.; Stokke, B.T. Probing macromolecular architectures of nanosized cyclic structures of (1→3)-β-d-glucans by afm and SEC-MALLS. Carbohyd. Res. 2005, 340, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.R.; Roberts, C.J.; Tendler, S.J.B.; Davies, M.C.; Williams, P.F. A C-13 CP MAS NMR spectroscopy and AFM study of the structure of glucagel™, a gelling β-glucan from barley. Carbohyd. Res. 1999, 315, 169–179. [Google Scholar] [CrossRef]
- Stokke, B.T.; Falch, B.H.; Dentini, M. Macromolecular triplex zipping observed in derivatives of fungal (1→3)-β-d-glucans by electron and atomic force microscopy. Biopolymer 2001, 58, 535–547. [Google Scholar] [CrossRef]
- Agbenorhevi, J.K.; Kontogiorgos, V.; Kirby, A.R.; Morris, V.J.; Tosh, S.M. Rheological and microstructural investigation of oat β-glucan isolates varying in molecular weight. Int. J. Biol. Macromol. 2011, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Synytsya, A.; Gedeon, O.; Slepicka, P.; Prochazka, V.; Synytsya, A.; Blahovec, J.; Hejlova, A.; Copikova, J. Yeast β(1–3),(1–6)-d-glucans films: Preparation and characterization of some structural and physical properties. Carbohyd. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Cricenti, A.; Generosi, R. Air operating atomic force-scanning tunneling microscope suitable to study semiconductors, metals, and biological samples. Rev. Sci. Instrum. 1995, 66, 2843–2847. [Google Scholar] [CrossRef]

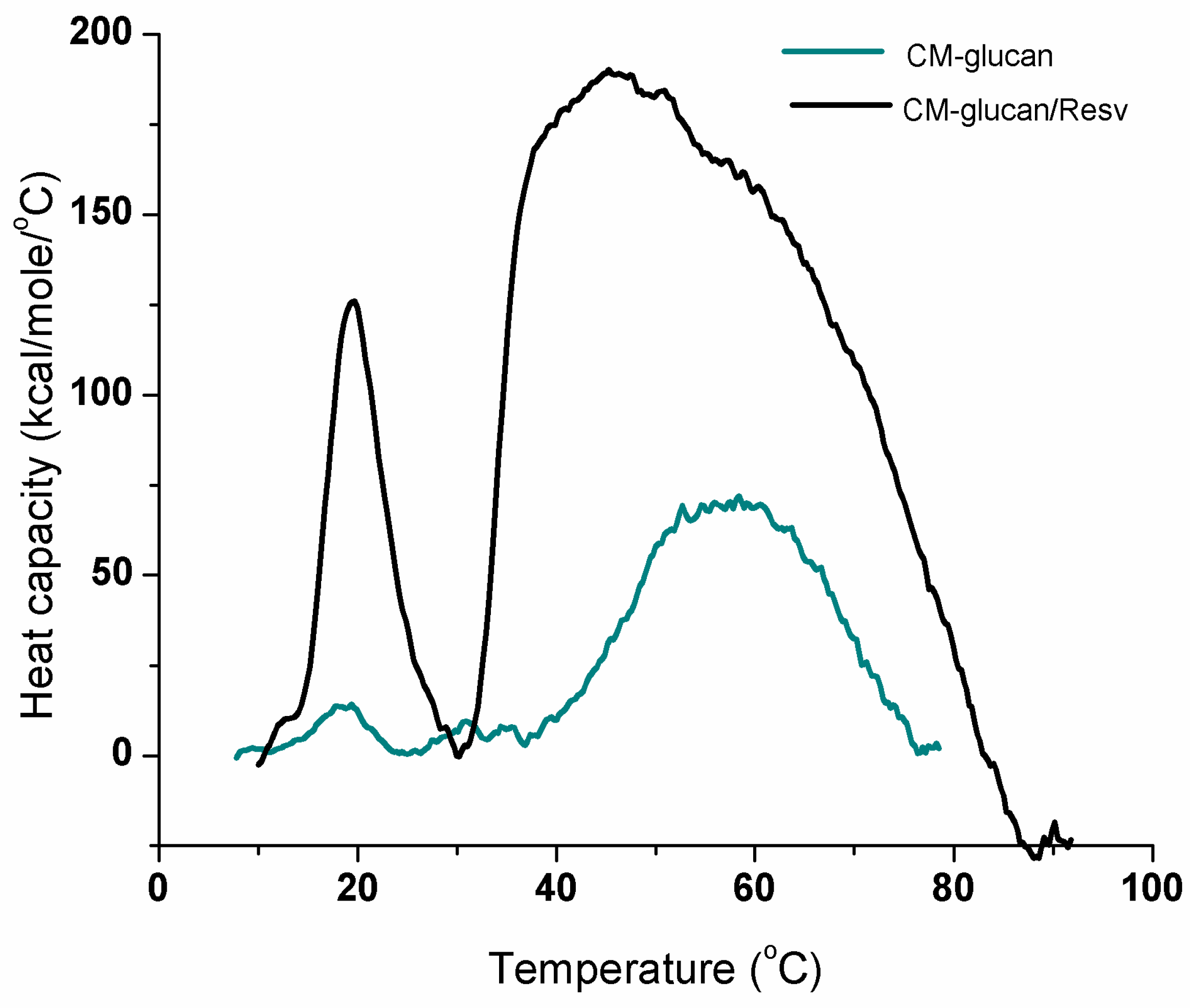



| Sample | Tm °C | ΔHcal kcal/mol | ΔHvH kcal/mol |
|---|---|---|---|
| CM-glucan | 19.0 | 78.1 | 117.9 |
| CM-glucan/resveratrol | 19.2 | 409.4 | 123.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francioso, A.; Dinarelli, S.; Girasole, M.; Cervoni, L.; D’Erme, M.; Mura, F.; Boffi, A.; Montanari, E.; Mosca, L. Behind Resveratrol Stabilization by Carboxymethylated (1,3/1,6)-β-d-Glucan: Does the Polyphenol Play a Role in Polymer Structural Organization? Int. J. Mol. Sci. 2017, 18, 2006. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18092006
Francioso A, Dinarelli S, Girasole M, Cervoni L, D’Erme M, Mura F, Boffi A, Montanari E, Mosca L. Behind Resveratrol Stabilization by Carboxymethylated (1,3/1,6)-β-d-Glucan: Does the Polyphenol Play a Role in Polymer Structural Organization? International Journal of Molecular Sciences. 2017; 18(9):2006. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18092006
Chicago/Turabian StyleFrancioso, Antonio, Simone Dinarelli, Marco Girasole, Laura Cervoni, Maria D’Erme, Francesco Mura, Alberto Boffi, Elita Montanari, and Luciana Mosca. 2017. "Behind Resveratrol Stabilization by Carboxymethylated (1,3/1,6)-β-d-Glucan: Does the Polyphenol Play a Role in Polymer Structural Organization?" International Journal of Molecular Sciences 18, no. 9: 2006. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18092006